Adv. Cardiol., vol. 25, pp. 9-24 (Karger, Basel 1978)
Minerals, Coronary Heart Disease and Sudden Coronary
Death
Supported in part by the Yrjö Jahnsson Foundation,
Helsinki, and Medipolar Oy, Oulu (Finland).
A preliminary report of part of this work has been published
[25].
H. KARPPANEN, R. PENNANEN and L. PASSINEN
Department of Pharmacology, University of Oulu, Oulu
Introduction
The high incidence of some diseases in certain geographical
areas can be explained by particular geological environments
[66]. Endemic goitre due to iodine deficiency in the soil is a
well-known example of such an 'environmental or geological
disease'. Great regional difference in the incidence of coronary
heart disease (CHD) suggest that the causes could be
environmental.
The interest in the possible role of various minerals in CHD
was mainly created by reports that regional death rates from
cardiovascular diseases in several countries are inversely
related to the hardness and thus to the mineral content of the
local drinking water [1-4, 9, 13-16, 28, 38-40, 42, 51, 52, 62,
67, 76]. Interestingly, goitre in Finland had its highest
incidence in the same areas where the death rates from CHD are
highest [70].
The high overall incidence, as well as the great regional
differences in the incidence of CHD in Finland (fig. 1) offer
good opportunities for studying the possible relation of
geological factors to CHD.
In the present work, correlations between the hardness of
drinking water and death rates from CHD in Finnish municipalities
were studied. The soil contents of some minerals were also
compared between the areas with the highest and lowest death
rates from CHD in Finland.
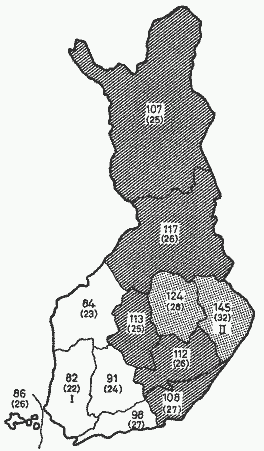
Fig. 1. Relative age-adjusted death rates from CHD in
different parts of Finland. The mortality rates are related to
average mortality from CHD in the whole country (= 100). From
PUSKA [46], by permission.

Water Hardness and Death Rates from CHD in Finnish
Municipalities
The results of determinations of water hardness during the
years 1971-1974 were obtained from all Finnish towns and some
bigger population centres. The arithmetic mean of the
determinations served as the hardness value for each
municipality. Official statistics from the years 1969-1972 were
used to calculate the death rates from acute or subacute CHD.
There is an inverse correlation between the hardness of
drinking water and the death rates from CHD in Finnish
municipalities (table 1). The linear dependence was statistically
significant only for men in the age group 25-64 years. However,
the correlation was statistically significant both for men and
women in the 25-54 years age group as well as between 25-64
years, when the death rates were plotted against the logarithm of
the hardness of water. Thus, the inverse statistical correlation
between the hardness of drinking water and death rate from CHD
was demonstrated also in Finland.
Soil Contents of Minerals in Finland
In Finland the contents of various minerals in arable soils
have been extensively determined for agricultural purposes [32].
The comparison between the areas with the lowest (area 1) and
highest mortality (area 11) from CHD (fig. 1) shows that in the
high mortality area in eastern Finland the soil contents of
calcium, magnesium and potassium are lower than in the
south-western area (table II). The difference is most pronounced,
2.7-fold, in the content of exchangeable magnesium. Also, over
the whole country there appears to be an inverse correlation
between the mortality from CHD and the soil content of magnesium.
The soil contents of potassium and calcium show a very similar
pattern to magnesium, but the differences between various parts
of the country are not so marked as in the case of magnesium
[32]. The contents of copper and boron are, on the average, very
similar in the two areas, whereas the content of manganese is
higher in east Finland than in the south-west (table II).
Based on a smaller material it has also been reported that the
soil content of selenium is lower in eastern Finland than in the
other parts of the country. Even in the latter areas soil,
selenium appears to be low in comparison with other countries
(29]. Furthermore, in well-water samples the contents of chromium
[44] and silicon [56] are lower in eastern Finland than in the
west.

Relation of Water Hardness to Sudden Coronary Death
ANDERSON et al. [2, 3] in Canada ascribed the higher
death rates in soft-water areas entirely to an excess of sudden
deaths due to an increased susceptibility to lethal arrhythmias
among residents of soft-water areas. A study in USA, which
defined sudden death as occurring at home or on arrival at
hospital, also indicated that a greater proportion of
cardiovascular deaths were sudden in the towns with soft-water
supplies [42]. Recently, CRAWFORD et al. [15]in England
and Wales reported a higher death rate from all cardiovascular
diseases including sudden deaths in the soft-water towns when
subjects were classified by age or social class, and also during
both winter and summer.
Possible Mechanisms of Action of Water Hardness and Soil
Minerals in CHD
Provided that the correlation between water hardness and soil
minerals on one hand and death rates from CHD on the other hand
is causal and not a spurious one, one or more of the following
mechanisms could increase mortality: (1) absolute decrease in the
intake of cardioprotective minerals; (2) absolute increase in the
intake of cardiotoxic minerals; (3) relative changes in the
intake of minerals in favour of cardiotoxic factors.
Possible Cardioprotective Minerals
Calcium and magnesium are the main elements
contributing to the hardness of the water. As calcium is usually
present in larger amounts, there has been more attention paid to
the possibility that calcium is the protective 'water factor'
[62]. Inverse correlations between the calcium content of water
and CHD, between dietary calcium intake and CHD, and as well with
total mortality have been observed [18, 27].
An increase in dietary calcium can lead to a decrease in serum
lipids, probably by the formation of insoluble soaps in the gut
[77]. Calcium may also compete with lead for absorption from the
gut. This has been advocated as an alternative mechanism by which
a high intake of calcium may decrease mortality [27].
Under certain circumstances high intakes of calcium may
increase rather than decrease the death rate from CHD [71]. There
is a highly significant positive correlation between death rates
from CHD and estimated calcium to magnesium ratios of the average
diets in OECD countries (fig. 2).

Fig. 2. Relationship between death rates from CHD and
the average calcium to magnesium ratio of the diet in different
OECD countries. Data adapted from KEYS [26] and VARO [71].
Since the daily intake of calcium is higher in Finland than in
most other countries [71] the high death rate from CHD in this
country cannot be attributed to a deficiency of calcium.
There is plenty of experimental, clinical and epidemiological
evidence to suggest that magnesium may have important
implications in CHD and sudden coronary death. Magnesium is one
of the major inorganic constituents of the cell. It is essential
for the activation of many enzymes and its plasma concentration
in relation to that of calcium affects nerve transmission and
muscular contraction. Magnesium participates in many of the most
vital oxidative, synthetic and transport processes of the
myocardial cells [43].
In clinical practice great emphasis is placed on potassium
loss in the etiology and intensification of cardiac arrhythmias
and cardiomyopathy. Recent evidence indicates that magnesium loss
may also be an important factor in these conditions. Magnesium is
necessary for the integrity of the mitochondrial system, which is
responsible for the maintenance of cellular potassium as well as
for the metabolic, processes [59]. Magnesium loss from the heart,
as from other tissues, interferes with the cellular machinery,
without which cardiac potassium cannot be maintained [59].
Magnesium loss thus predisposes to potassium loss.
Recent studies indicate that cellular loss of magnesium may be
a basic biochemical mechanism in the evolution of arrhythmias and
myocardial lesions of diverse etiology. Net loss of magnesium
from the myocardium is a well-known phenomenon associated with
oxygen deficiency. Loss of magnesium from hypoxic myocardium has
been found both in several cardiomyopathic animal models and in
patients who have died of myocardial infarcts [43, 59, 60, 62].
An early elevation of myocardial calcium is associated with a
fall of myocardial magnesium both in experimental models of
myocardial infarction and in human sufferers [59, 60, 62].
High doses of catecholamines cause myocardial necrosis, which
is preceded by an elevation of myocardial calcium and a drop in
myocardial magnesium [33], as well as a serious fall in the
myocardial content of high-energy phosphates [19, 20]. These
catecholamine effects are particularly interesting since
psychogenic factors [49, 50] and the sympathetic nervous system
[72] appear to be involved in sudden coronary death.
Both the fall of magnesium and elevation of calcium, which are
associated with myocardial damage, may increase cellular
excitability, thereby contributing to the development of
ventricular arrhythmias [62].
Dietary magnesium deficiency in animals predisposes to
ventricular extrasystoles (30, 73] and myocardial damage
[69].
Prolonged therapy with thiazide diuretics is known to induce,
in addition to hypokalemia, a marked magnesium deficiency in a
considerable proportion of patients [17, 34]. Loss of magnesium
and potassium and retention of calcium by thiazide diuretics
increase the risk of arrhythmias [17]. This might partly explain
why the treatment of hypertension has not diminished the death
rate from CHD, in spite of marked reduction in other
complications. It has been suggested that patients on diuretics
should be given magnesium together with potassium supplements
[17]. The administration of magnesium is especially important,
since in magnesium deficiency there is refractoriness to
potassium repletion [75]. Moreover, administration of magnesium
has also been shown to restore potassium stores [34].
On the basis of metabolic balance studies it has been
concluded that occidental magnesium intakes are suboptimal [57,
69]. The amount of magnesium provided by hard water may be
sufficient to correct a marginal deficit, thereby contributing to
the lower death rates from ischemic heart disease in hard-water
than in soft-water areas [62]. Residents of soft-water areas have
lower magnesium concentrations in heart muscle [4] and coronary
arteries [141 than do residents of hard-water areas. Moreover, a
diminished content of myocardial magnesium has been found after a
sudden death from heart disease [11].
The results of an exhaustive work by ALLEN [1] in Ontario,
Canada, suggest that the protective effect of hard water in CHD
is due to the magnesium component. Magnesium was more effective
than total hardness, which in turn was more effective than
calcium in favourably influencing the rate of sudden deaths from
CHD. The role of magnesium in maintaining a normal rhythm of the
heart in the face of an ischemic insult might explain the
difference in sudden cardiac death rates between hard and
soft-water areas [62].
The average content of magnesium in Finnish soils is low [31,
32]. This is especially true for the eastern areas where the
death rates from CHD are very high. The low soil content is
reflected in a very low content of magnesium in the drinking
water and a lower intake of magnesium in eastern Finland compared
with the south-western parts of the country.
In the past half century, magnesium intakes have fallen,
whereas dietary contents of protein, fat, sugar, and calcium have
risen [61]. High intakes of these nutrients increase magnesium
requirements and increase susceptibility to magnesium deficit
[57, 58, 61, 62]. Due to the low content of magnesium in the
drinking water, and possibly also in agricultural products, such
a change may have more, serious consequence in eastern Finland
than in most other areas. Moreover, the consumption of milk
products is, on the average, higher in Finland than in any other
country [71]. The calcium to magnesium ratio in milk is about
12:1 [71]. The high calcium concentration in the average Finnish
diet is likely to inhibit the intestinal absorption of magnesium
[71] and also to increase the loss of magnesium [58]. Thus,
because of the geochemical environment and dietary habits the
occurrence of magnesium deficiency is more likely in Finland than
in most other countries. However, it should be emphasised that no
studies have been done to examine whether magnesium deficiency
does exist in Finland.
If the relation of magnesium depletion to cardiac arrhythmias
and myocardial damage is a causal one, magnesium administration
should have a beneficial effect in these conditions. In fact,
there is laboratory evidence that magnesium salts protect against
hypoxic damage to the heart [5, 10, 62]. Magnesium chloride and
calcium antagonists also protect against catecholamine-induced
myocardial damage [19]. In man, successful use of magnesium in
the treatment of cardiac arrhythmias of various etiologies has
been reported [34, 62, 63, 68, 78]. Correction of the magnesium
deficit protects against cardiovascular lesions in several
species of animals on atherogenic, magnesium-deficient diets [41,
62].
Potassium depletion predisposes to cardiac
arrhythmias. The soil content of potassium is lower in eastern
Finland than in Southwestern Finland, and potassium intake is
probably lower in the high-mortality area. Since monovalent
cations are easily absorbed from the gastrointestinal tract, a
primary dietary deficiency of potassium seems unlikely. Decreased
dietary intake is likely to predispose to potassium depletion in
cases of increased loss due to diuretic therapy or
gastrointestinal causes.
Little is known about the exact role of several other minerals
upon CHD and sudden death in man and further studies are needed
to establish their role. Chromium in cardiovascular
diseases has been reviewed earlier [53]. Chromium deficiency in
rats induces atherosclerosis, and results of epidemiological
studies indicate that chromium may also be related to
cardiovascular disease in man. In populations with low incidences
of cardiovascular diseases the chromium content of the heart,
kidney and aorta is many times higher than in populations with
high incidences of these diseases. Chromium is necessary for
glucose and lipid metabolism. The decreased or absent aortic
chromium in atherosclerosis may reflect the same thing in the
coronary arteries, which leads to abnormal metabolism and plaque
formation.
The concentration of chromium in drinking water was found to
be lower in the high-mortality area of eastern Finland compared
with the low-mortality area in the west Finland (44]. The urinary
excretion of chromium of the two populations was similar and no
consistent differences were found in urinary chromium excretion
between groups with atherosclerotic diseases and reference groups
[45]. In population studies in Canada and UK, chromium, either in
myocardial tissues or as a water constituent, has not been found
to be associated with increased CHD or sudden deaths [4, 18].
Selenium has proved to be cardioprotective in several
animal studies [21, 22, 37, 64, 65]. Death rates from
cardiovascular diseases have been reported to be lower in a very
high selenium area than in the low selenium area [651. The
selenium content of the Finnish soil is low [29]. The level of
selenium is also low in the blood of Finns, suggesting possible
selenium deficiency [74].
Results from silicon are contradictory. Silicon seems
to protect the elastic state of the artery walls and has been
claimed to maintain the intimal impermeable to lipid infiltration
[37]. Silicate-silicon may be the active agent in dietary fibre
[55] which affects the development of atherosclerosis. Water
appears to provide several times more silicon than food [531.
Drinking water in east Finland contains less silicon than in west
Finland [561.
A positive correlations between cardiovascular mortality and
silicon content in drinking water, consistent with a harmful
effect of this element, has been found [18]. Moreover, when
silicon in heart muscle from people who had died suddenly from
ischemic heart disease and from controls in the same area was
measured, it appeared that the cases of sudden death had higher
concentrations of silicon than the controls, though the
difference was not statistically significant [12].
Incidence and severity of aortic calcification have been found
to be lower in areas supplied with highly fluoridated
waters than in non-fluoridated areas [7]. Addition of fluoride
and magnesium to high sucrose, high phosphate diets prevented
aortic calcification in rats [361. A lower incidence of
cardiovascular disease in the areas with the highest
concentrations of fluoride and magnesium in the drinking water
compared with areas with low concentrations of these elements in
water is found [35]. However, the magnesium component may have
played an important role in these results. On the other hand, it
has been shown that high doses of fluorine produce focal
myocardial necroses and lesions of the cerebral cortex in the rat
[37].
Manganese has been reported to exert a beneficial
effect on lipid metabolism in atherosclerotic patients, and to
prevent the development of experimental atherosclerosis in
rabbits [37]. However, the content of manganese in soils is
higher in the high-mortality area of eastern Finland compared to
south-western Finland. Soft water also contains more manganese
than hard water [18].
Possible Cardiotoxic Minerals
When administered to rats in doses which produced the tissue
levels of cadmium found in North-American subjects,
cadmium produced hypertension, hypertrophy of the left ventricle
and sclerosis of the small arteries in kidneys, heart and other
organs [37]. A positive correlation between the cadmium content
in the air and the incidence of hypertension and atherosclerosis
in 28 North-American cities has been demonstrated. Cadmium is
present in high concentrations in the kidneys and urine of
hypertensive patients [53]. In Kansas City, Kansas, the content
of cadmium in drinking water is three times higher than in Kansas
City, Missouri. The higher content of cadmium in water was
associated with a tenfold higher content of this metal in serum,
significantly higher systolic and diastolic blood pressures, and
higher mortality from cardiovascular diseases in Kansas City,
Kansas, than in Kansas City, Missouri [8]. Due to corrosive
properties of soft water, cadmium is dissolved from pipes so that
content is usually higher in soft water than in hard [53].
Renal cadmium concentration is lower in populations relatively
unexposed to industrial surroundings. Hence it seems probable
that exposure to cadmium in the rural areas of eastern Finland is
not high. Furthermore, in these high mortality areas a big
proportion of drinking water is derived from private wells,
either via short water pipes or without pipes. In spite of the
water being soft, these local conditions diminish the risk of
waterborne exposure to cadmium. On the other hand, it has been
reported that the so-called 'muikku' fish (Coregonus
albula) which is heavily consumed in eastern Finland,
contains the highest cadmium concentration of all Finnish fish
products [24]. Another source of cadmium is cigarette smoke [54].
Men, but not women, in eastern Finland are heavy smokers [47].
This might partly explain the great difference in the death rates
from CHD between men and women in this area. However, the body
burden of cadmium in Finland is not known.
Lead is another metal which is present in higher
concentrations in soft water than in hard [18]. Fed in water to
rats, lead causes myocardial damage [18]. However, in South Wales
any connection between lead content in water and mortality seems
to be very slight [18].
Copper in the context of cardiac diseases has been
reviewed earlier [53]. Copper is present in higher concentrations
in soft water than in hard. In patients suffering from myocardial
infarction, serum copper was increased. In experimental animals
the, addition of copper to the diet resulted in a higher degree
of atherosclerosis. In atherosclerotic subjects the copper
content of the aorta wall was found to be decreased, while that
of the myocardium increased.
No significant correlation between the water content of copper
and cardiovascular mortality was found in England and Wales [18].
Also, the average soil content of copper in the areas with the
highest and lowest rates from CHD in Finland are similar. Thus,
at least in Finland, the regional differences in the death rates
from CHD can hardly be attributed to different intakes of
copper.
Conclusions
There is evidence that loss of myocardial magnesium and
potassium, and rise in myocardial calcium predispose to
ventricular arrhythmias and thus to sudden coronary death.
Myocardial depletion of magnesium and potassium may be due to
hypoxia, decreased dietary bioavailability, or increased loss of
these minerals. In experimental animals, dietary magnesium
deficiency also promotes the development of atherosclerosis.
Deficiency of several trace elements, such as chromium and
silicon, may also increase the risk of atherosclerosis. On the
other hand, an increased body burden of cadmium may predispose to
sudden coronary death by inducing hypertension.
In Finland the soil contents of several minerals, e.g. that of
magnesium, are very low, especially in the eastern areas where
the mortality rates from CHD are high. The low soil content of
mineral in eastern Finland could lead to deficiency states which
may be involved in the high mortality from CHD.
References
1 ALLEN, H.A.J.: An investigation of water hardness, calcium
and magnesium in relation to mortality in Ontario; thesis
University of Waterloo, Ontario (1972).
2 ANDERSON, T.W. and LE RICHE, W.H.: Sudden death from
ischemic heart disease in Ontario and its correlation with water
hardness and other factors. Can. med. Ass. J. 105:
155-160 (1971).
3 ANDERSON, T.W.; LE RICHE, W.H., and MACKAY, J.S.: Sudden
death and ischemic heart disease. Correlation with hardness of
local water supply. New Engl. J. Med. 280: 805-807
(1969).
4 ANDERSON, T.W.; NERI, L.C.; SCHREIBER, G.B.; TALBOT, F.D.F.,
and ZDROJEWSKI, A.: Ischemic heart disease, water hardness and
myocardial magnesium. Can. med. Ass. J. 113: 199-203
(1975).
5 BAJUSZ, E. and SELYE, H.: The chemical prevention of cardiac
necroses following occlusion of coronary vessels. Can. med. Ass.
J. 82: 212-213 (1960).
6 BALL, K. and TURNER, R.: Smoking and the heart: the basis
for action. Lancet ii: 822-826 (1974).
7 BERNSTEIN, D.S.; SADOWSKY, N.; HEGSTED, D.M.; GURI, C.D.,
and STARE, F.J.: Prevalence of osteoporosis in high- and
low-fluoride areas in North Dakota. J. Am. med. Ass.
198: 499-504 (1966).
8 BIERENBAUM, M.L.; FLEISCHMAN, A.I.; DUNN, J., and ARNOLD,
J.: Possible toxic water factor in coronary heart-disease. Lancet
i: 1008-1014 (1975).
9 BIÖRCK, G.; BOSTRÖM, H., and WIDSTRÖM, A.: On
the relationship between water hardness and death rate in
cardiovascular diseases. Acta med. scand. 178: 239-251
(1965).
10 CARDEN, N.L. and STEINHAUS, J.R.: Role of magnesium ion in
the initiation of ventricular fibrillation produced by acute
coronary occlusion. Circulation Res. 5: 405-408
(1957).
11 CHIPPERFIELD, B. and CHIPPERFIELD, J.R.: Heart-muscle
magnesium, potassium, and zinc concentrations after sudden death
from heart disease. Lancet ii: 293-296 (1973).
12 CHIPPERFIELD, B.; CHIPPERFIELD, J.R., and BOWER, N.R.:
Silicon and aluminium in heart deaths. Lancet i: 755-756
(1977).
13 CRAWFORD, M.D.; CLAYTON, D.G.; STANLEY, F., and SMKPFR,
A.G.: An epidemiological study of sudden death in hard and soft
water areas. J. chron. Dis. 30: 69-80 (1977).
14 CRAWFORD, T. and CRAWFORD, M.D.: Prevalence and
pathological changes of ischaemic heart disease in a hard-water
and in a soft-water area. Lancet i: 229-232 (1967).
15 CRAWFORD, M.D.; GARDNER, M.J., and MORRIS, J.N.: Mortality
and hardness of local water-supplies. Lancet i: 827-831
(1968).
16 CRAWFORD, M.D.; GARDNER, M.J., and MORRIS, J.N.:
Cardiovascular disease and the mineral content of drinking water.
Br. med. Bull. 27: 21-24 (1971).
17 EDITORIAL: Calcium, magnesium, and diuretics. Br. med. J.
i: 170-171 (1975).
18 ELWOOD, P.C.; ABERNETHY, M., and MORTON, M.: Mortality in
adults and trace elements in water. Lancet ii: 1470-1472
(1974).
19 FLECKENSTEIN, A.: Specific inhibitors and promoters of
calcium action in the excitation-contraction coupling of heart
muscle and their role in the prevention or production of
myocardial lesions; in HARRIS and OPIE Calcium and the heart, pp.
135-188 (Academic Press, London 1971).
20 FLECKENSTEIN, A.; JANKE, J.; DÖRING, H.J., and LEDER,
O.: Myocardial fiber necrosis due to intracellular Ca overload -
a new principle in cardiac pathophysiology; in DHALLA Recent
advances in studies on cardiac structure and metabolism, vol. 4:
Myocardial biology, pp. 563-580 (University Park Press, Baltimore
1974).
21 GODWIN, K.O.: Abnormal electrocardiograms in rats fed a
low-selenium diet. Q. JI exp. Physiol. 50: 282-288
(1965).
22 GODWIN, K.O. and FRASER, F.J.: Abnormal electrocardiograms,
blood pressure changes, and some aspects of the histopathology of
selenium deficiency in lambs. Q. JI exp. Physiol. 51:
94-102 (1966).
23 JANKE, J.; FLECKENSTEIN, A.; HEIN, B.; LFDER, O., and
SIGEL, H.. Prevention of myocardial Ca overload and necrotization
by Mg and K salts or acidosis; in FLECKENSTEIN and RONA Recent
advances in studies on cardiac structure and metabolism, vol. 6:
Pathophysiology and morphology of myocardial cell alteration, pp.
33-42 (University Park Press, Baltimore 1975).
24 KARPPANEN, E. and STABEL-TAUCHER, R.: Suomen
vesistöjen kalojen ja kaupan olevien kalasäilykkeiden
metallipitoisuuksia. Suornen
Eläinlääkärilehti 82: 277-284
(1976).
25 KARPPANEN, H. and NEUVONEN, P.J.: Ischaemic heart-disease
and soil magnesium in Finland. Lancet ii: 1390
(1973).
26 KEYS, A. (ed.). Coronary heart disease in seven countries.
Am. Heart Ass. Monogr. No. 29, New York (1970).
27 KNOX, E.G.: Ischaemic heart disease mortality and dietary
intake of calcium. Lancet i: 1465-1467 (1973).
28 KOBAYASHI, J.: On geographical relationship between the
chemical nature of river water and death rate from apoplexy. Ber.
Öhara Inst. landw. Forsch. 11: 12-21 (1957).
29 KOLJONEN, T.: The behavior of selenium in Finnish soils.
Ann. Agric. Fenn. 14: 240-247 (1975).
30 KRUSE, H.D.; ORENT, E.R., and MCCOLLUM, E.V.: Studies on
magnesium deficiency in animals. I. Symptomatology resulting from
magnesium deprivation. J. biol. Chem. 96: 519-539
(1932).
31 KURKI, M.: Viljelysmaiden magnesiumtilanne
(Viljavuuspalvelu Oy, Helsinki 1971).
32 KURKI, M.: Suomen peltojen viljavuudesta II
(Viljavuuspalvelu Oy, Helsinki 1972).
33 LEHR, D.; CHAU, R., and IRENE, S.: Possible role of
magnesium loss in the pathogenesis of myocardial fiber necrosis;
in FLECKENSTEIN and RONA Recent advances in studies on cardiac
structure and metabolism, vol. 6: Pathophysiology and morphology
of myocardial cell alteration, pp. 95-109 (University Park Press,
Baltimore 1975).
34 Lim, P. and JACOB, E.: Magnesium deficiency in patients on
long-term diuretic therapy for heart failure. Br. med. J.
iii: 620-622 (1972).
35 LUOMA, H.; HELMINEN, S.K.J.; RANTA, H.; RYTÖMAA, I.,
and MEURMAN, J.H.: Relationships between the fluoride and
magnesium concentrations in drinking water and some components in
serum related to cardiovascular diseases in men from four rural
districts in Finland. Scand. J. clin. Lab. Invest. 32:
217-224 (1973).
36 LUOMA, H.; NUUJA, T.; COLLAN, Y., and NUMMIKOSKI, P.: The
effect of magnesium and fluoride on nephrocalcinosis and aortic
calcification in rats given high sucrose diets with added
phosphates. Calcif. Tiss. Res. 20: 291-302 (1976).
37 MASIRONI, R.: Trace elements and cardiovascular diseases.
Bull. WHO 40: 305-312 (1969).
38 MORRIS, J.N.; CRAWFORD, M.D., and HEADY, J.A.: Hardness of
local water supplies and mortality from cardiovascular disease in
the county boroughs of England and Wales. Lancet i:
860-862 (1961).
39 MORRIS, J.N.; CRAWFORD, M.D., and HEADY, J.A.: Hardness of
local water supplies and mortality from cardiovascular disease.
Lancet ii: 506-507 (1962).
40 MULCAHY, R.: Mortality and hardness of water-supplies.
Lancet i: 975 (1968).
41 NEAL, J. and NEAL, M.: Effect of hard water and
MgSO4 on rabbit atherosclerosis. Archs Path.
73: 400-403 (1962).
42 PETERSON, D.R.; THOMPSON, D.J., and NAM, J.M.: Water
hardness, arteriosclerotic heart disease and sudden death. Am. J.
Epidem. 92: 90-93 (1970).
43 POLIMENI, P.I. and PAGE, E.: Magnesium in heart muscle.
Circulation Res. 33: 367-374 (1973).
44 PUNSAR, S.; ERÄMETSÄ, O.; KARVONEN, M.J.;
RYHÄNEN, A.; HILSKA,, P., and
VORNAMO, H.: Coronary heart disease and drinking water. A
search in two Finnish male cohorts for epidemiologic evidence of
a water factor. J. chron. Dis. 28: 259-287 (1975).
45 PUNSAR, S.; WOLF, W.; MERTZ, W., and KARVONEN, M.J.:
Urinary chromium excretion and atherosclerotic manifestations in
two Finnish male populations. Ann. clin. Res. 9: 79-83
(1977).
46 PUSKA, P.: Sydän- ja verisuonisairauksien aiheuttaman
kuolleisuuden alueelliset erot. I Tilanne vuoden 1969 tilastojen
valossa. Suomen Lääkärilehti 27:
3071-3075 (1972).
47 RUMPELÄ, M.; PUSKA, P.; SILVERS, K.; TUOMILEHTO, J.;
VIRTAMO, J.; KARJALAINEN, Y., and PRUNNILA, T.: The baseline
survey of North Karelia Project: study design and prevalence of
major CHD risk-indicators. Sos.Iääket. Aikak.I.
2: 97-106 (1974).
48 ROMO, M.: Factors related to acute ischemic heart disease.
A community study in Helsinki. Acta med. scand., suppl. 547, pp.
1-87 (1973).
49 ROSENMAN, R.H.: The role of type A behavior pattern in
pathogenesis of ischemic heart disease and modification for
prevention (this volume).
50 ROSENMAN, R.H. and FRIEDMAN, M.: Neurogenic factors in
pathogenesis of coronary heart disease. Med. Clin. N. Am.
58: 269-279 (1974).
51 SCHROEDER, H.A.: Relation between mortality from
cardiovascular disease and treated water supplies. Variations in
states and 163 largest municipalities of the United States. J.
Am. med. Ass. 172: 1902-1908 (1960).
52 SCHROEDER, H.A.: Municipal drinking water and
cardiovascular death rates. J. Am. med. Ass. 195:
125-129 (1966).
53 SCHROEDER, H.A.: The role of trace elements in
cardiovascular diseases. Med. Clins N. Am. 58: 381-396
(1974).
54 SCHROEDER, H.A. and BALASSA, J.J.: Abnormal trace metals in
man: Cadmium. J. chron. Dis. 14: 236-258 (1961).
55 SCHWARZ, K.: Silicon, fibre, and atherosclerosis. Lancet
i: 454-457 (1977).
56 SCHWARZ, K.; RICCI, B.A.; PUNSAR, S., and KARVONEN, M.J.:
Inverse relation of silicon in drinking water and atherosclerosis
in Finland. Lancet i: 538-539 (1977).
57 SEELIG, M.S.: The requirement of magnesium by the normal
adult. Am. J. clin. Nutr. 14: 342-390 (1964).
58 SEELIG, M.S.: Human requirements of magnesium: factors that
increase needs; in DURLACH ler Symp. int. sur le déficit
magnésique en pathologie humaine, vol. 1, pp. 11-38
(Vittel 1971).
59 SEELIG, M.S.: Myocardial loss of functional magnesium. 1.
Effect on mitochondrial integrity in studies on cardiac structure
and metabolism; in BAJUSZ and RONA Myocardiology, vol. 1, pp.
615-625 (University Park Press, Baltimore 1972).
60 SEELIG, M.S.: Myocardial loss of functional magnesium. II.
In cardiomyopathies of diverse etiology; in BAJUSZ and RONA
Recent advances in studies on cardiac structure and metabolism,
Myocardiology, vol. 1, pp. 626-638 (University Park Press,
Baltimore 1972).
61 SEELIG, M.S.: Ischaemic heart disease, vitamins D and A,
and magnesium. Br. med. J. iii: 647-648 (1975).
62 SEELIG, M.S. and HEGGTVEIT, H.A.: Magnesium
interrelationships in ischemic heart disease: a review. Am. J.
clin. Nutr. 27: 59-79 (1974).
63 SELLER, R.H.: The role of magnesium in digitalis toxicity.
Am. Heart J. 82: 551-556 (1971).
64 SELYE, H.: Chemical prevention of cardiac necroses (Ronald
Press, New York 1958).
65 SHAMBERGER, R.J.; TYTKO, S.A., and WILLIS, C.E.: Selenium
and heart disease; in HEMPHILL Trace substances in environmental
health, vol. 9,: pp. 15-22 (University of Missouri Press,
Columbia 1975).
66 SPENCER, J.M.: Geological influence on regional health
problems. Texas J. Sci. 21: 459-469 (1970).
67 STITT, F.W.; CLAYTON, D.G.; CRAWFORD, M.D., and MORRIS,
J.N.: Clinical and biochemical indicators of cardiovascular
disease among men living in hard and soft water areas. Lancet
i: 122-126 (1973).
68 SZEKELY, P. and WYNNE, N.A.: The effects of magnesium on
cardiac arrhythmias caused by digitalis. Clin. Sci. 10:
241-253 (1951).
69 SZELÉNYI, I.: Magnesium and its significance in
cardiovascular and gastrointestinal disorders. Wld. Rev. Nutr.
Diet., vol. 17, pp. 189-224 (Karger, Basel 1973).
70 UOTILA, U.; RAEKALLIP, J., and EHRNROOTH, W.: Goitre and
arteriosclerosis. Lancet ii; 171-173 (1958).
71 VARO, P.: Mineral element balance and coronary heart
disease. Int.J. Vit. Nutr. Res. 44: 267-273 (1974).
72 VERRIER, R.: Higher neural activity and sudden death (this
volume).
73 VITALE, J.; HELLERSTEIN, E.; NAKAMURA, M., and LOWN, B.:
Effects of magnesium deficient diet upon puppies. Circulation
Res. 9: 387-394 (1961).
74 WESTERMARCK, T.; RAUNU, P.; KIRJARINTA, M., and
LAPPALAINEN, L.: Selenium content of whole blood and serum in
adults and children of different ages from different parts of
Finland. Acta pharmac. tox. 50: 465-475 (1977).
75 WHANG,,R. and AIKAWA, J.K.: Magnesium deficiency and
refractoriness to potassium repletion. J. chron. Dis.
30: 65-68 (1977).
76 WHO: Water quality, trace elements, and cardiovascular
disease. WHO Chron. 27: 534-538 (1973).
77 YACOWITZ, H.; FLEISCHMAN, A.I., and BIERENBAUM, M.L.:
Effects of oral calcium upon serum lipids in man. Br. med. J.
i: 1352-1354 (1965).
78 ZWILLINGER, L.: Über die Magnesiumwirkung auf das
Herz. Klin. Wschr. 14: 1429-1433 (1935).
H. KARPPANEN, MD, Department of Pharmacology, University
of Oulu, SF-90100 Oulu 10 (Finland)
This page was first uploaded to The Magnesium Web Site on
March 8, 2002
http://www.mgwater.com/




