The Magnesium Report
Clinical, Research, and Laboratory News for Cardiologists
August 1999
Oral Magnesium in Coronary Artery Disease:
Fresh Insight on Thrombus Inhibition
MICHAEL SHECHTER, MD, MA
Despite enormous strides in the understanding, prevention, and
treatment of coronary artery disease (CAD) and acute myocardial
infarction (MI) over the past 20 years, CAD remains the leading
cause of death for both men and women in the United States. The
management of acute MI now routinely involves a complex array of
effective interventions including reperfusion therapy and
cardioprotective and antithrombogenic agents. Yet both morbidity
and mortality remain unacceptably high, particularly in the
elderly.
Promise of Magnesium
Magnesium continues to undergo study as a cardioprotective
agent. In acute MI, it is relatively safe, inexpensive, and easy
to use either as an adjunct or an alternative to thrombolytics
and other agents.
Magnesium, nature's physiologic calcium channel blocker, is
the second most common intracellular cation in the human body
after potassium) and a crucial cofactor for many physiologic
processes. Although clinical trials of intravenous (IV) magnesium
in acute MI have yielded conflicting results, magnesium has
demonstrated antiarrhythmic, antithrombogenic, and vasodilating
effects that have limited infarct size and protected against
reinfarction.
Recent evidence points to a beneficial role for oral magnesium
as a regulator of platelet dependent thromboses (PDT). In a study
of CAD patients published in the July 15, 1999, issue of the
American Journal of Cardiology described below, my
colleagues and I discovered PDT was reduced by 35% in patients
who received oral magnesium supplementation.
Magnesium, CAD and MI
There is impressive evidence that magnesium deficiency
participates in the pathogenesis of CAD. Magnesium supplements
have reduced development of atherosclerosis in animal studies.
Magnesium deficiency has been associated with several important
CAD risk factors including hypertension, diabetes mellitus,
dyslipidemia, arrhythmia, coronary vasospasm, and thrombosis.
The first epidemiological links between magnesium status and
CAD were the observations that mortality from ischemic heart
disease was lower in populations living in areas with "hard"
water than in "soft" water regions. Hardness of water is caused
by high concentrations of both calcium and magnesium, but
evidence has shown that protection against cardiovascular damage
is afforded primarily by magnesium, not calcium. Autopsies of
patients in soft water areas have shown more coronary atheromata
and evidence of old and recent MI, and lower levels of magnesium
in cardiac tissue.
Low serum magnesium concentrations at admission have been
reported in many patients with acute MI, but not in all. Acute
depression of serum magnesium levels is accompanied by an
increase in free fatty acid levels triggered by release of
catecholamines that also lower tissue magnesium levels. Acute and
chronic reduction in extracellular magnesium levels is associated
with lowered myocardial magnesium, which increases the risk of
damage from myocardial ischemia.
Magnesium deficiency, demonstrated better by mononuclear blood
cell magnesium that serum level [Elin RJ. Magnesium metabolism in
health and disease. Dis.
Mon.;34:1-218.], predisposes to excessive
morbidity or mortality of acute MI patients. These patients have
been shown to have low mononuclear blood cell magnesium, which is
predictive of in-hospital mortality: the lower the magnesium
level, the higher the mortality. Dietary magnesium intake was
found to be lower in patients who had ventricular arrhythmia
during acute MI. Some laboratory and clinical trials suggest that
magnesium can reduce total and low-density lipoprotein
cholesterol (LDL-C) and increase high-density lipoprotein
cholesterol (HDL-C).
The Thrombogenesis Connection
The benefits of IV magnesium infusion treatment of acute MI
patients remain controversial, having been demonstrated in some
randomized, controlled clinical trials but not others. My
colleagues and I demonstrated that IV magnesium therapy reduces
mortality in the thrombolysis-ineligible patients with acute MI,
including the elderly. However, a multicenter megatrial showed no
benefit and even a nonsignificant trend toward harm.
Why have trials of magnesium therapy in MI produced
inconsistent results? A plausible explanation is that trials
administer magnesium at different times after infarction. Best
results are obtained with the least delay in infusing magnesium
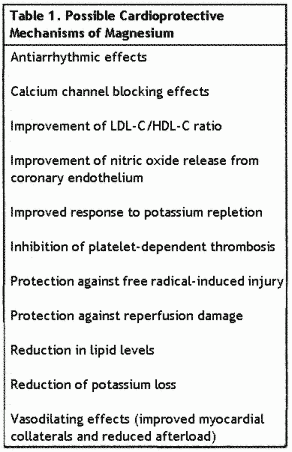
after the ischemic event. Table 1 lists the known physiologic
activities of magnesium that provide clues to its protective
effects. The cascade and its suppression of platelet activation
have immediate and longer-term benefits in CAD
patients.
Current Study Results. To test our
hypothesis that magnesium treatment plays a regulating role in
thrombogenesis, we recently conducted a randomized, prospective,
double-blind, crossover trial of the effects of oral magnesium
supplementation on PDT in 42 patients (37 men, 5 women, mean age
68±9 years) with documented CAD, all of whom were taking
aspirin. Excluded from the study were patients with unstable
angina, severe congestive heaart failure (CHF), chronic diarrhea,
renal failure, MI, within the preceding 3 months, abnormal
thyroid function, peripheral vascular disease, chronic liver
disease, or type 1 (insulin-dependent) diabetes mellitus. In the
study population, 55% of the patients had prior MI, 75% had a
history of hypercholesterolemia, 58% had systemic hypertension,
and 6% had type 2 (non-insulin-dependent) diabetes mellitus.
Patients received either magnesium oxide tablets (800 to 1200
mg/d [483 to 724 mg elemental magnesium]) or placebo for 3
months, followed by a 4-week washout period, and then crossover
treatment for 3 months. Subjects continued taking their regular
medications, which included aspirin and various combinations of
beta blockers, lipid-lowering agents, ACE inhibitors, calcium
antagonists, and diuretics. Before and after each phase, PDT was
assessed using an ex vivo perfusion model (porcine aortic media
held in a Badimon chamber). Other measured parameters included
platelet aggregation, platelet P-selectin flow-cytometry,
monocyte tissue factor procoagulant activity (TF-PCA), adhesion
molecule density, and serum lipids and electrolytes.
Compliance with the study medication was 89%, and no serious
adverse effects were reported. Among the 36 subjects included in
the final analysis, no significant differences were found in
baseline values before each treatment period. Before treatment
began, baseline analysis revealed significant positive
correlations between PDT and fasting blood sugar (r =
0.44), resting systolic blood pressure (r = 0.37),
apolipoprotein B (r = 0.29%), and total cholesterol
level (r = (0.42). No significant correlation was
observed between PDT and TF-PCA, either at baseline or following
treatment.
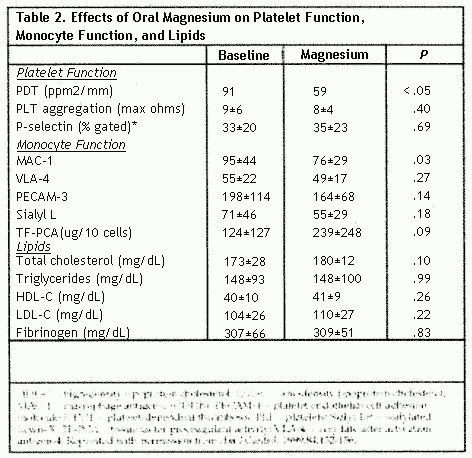
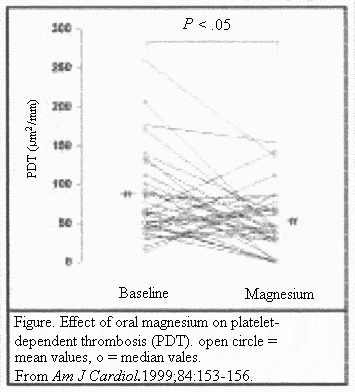
After 3 months of oral magnesium supplementation, the median
PDT was reduced by 35% (P<.05) (Table 2), and 75% of
the patients demonstrated a decrease in PDT with magnesium oxide
treatment (P<.05) (Figure) No association was
observed between the presence of risk factors (hypertension,
smoking, diabetes mellitus) and the degree of change in PDT after
treatment. Magnesium treatment produced no significant effect on
platelet aggregation. P-selection expression, monocyte-derived
TF-PCA, serum lipids, fibrinogen, or apolipoprotein adhesion
molecules, MAC-1 was significantly reduced (P<.03)
during treatment.
Only 1 patient had a subnormal serum magnesium level at
baseline (1.6 mg/dL). After 3 months of oral magnesium treatment,
there was no significant change in serum magnesium levels in the
study population (2.08±0.16 vs 2.11±0.14 mg/dL,
P=.22). Nor was there any correlation between baseline
serum magnesium levels and PDT or between the change in serum
magnesium levels and the change in PDT. However, the increase in
magnesium level after treatment was significantly greater when
baseline levels were low (r = 0.53,
P=.0008).
Implications for Treatment
Our recent study demonstrated for the first time that an ex
vivo measure of acute PDT was significantly reduced in stable CAD
patients receiving oral magnesium therapy. This antithrombotic
effect occurred despite 100% use of aspirin therapy. Magnesium
therapy did not inhibit either platelet aggregation or P-selectin
expression, a measure of platelet α-granule release
reaction.
We observed no effect on collagen-induced platelet aggregation
at an oral magnesium dose that caused significant inhibition of
platelet adhesion and thrombus formation. Several factors may
explain why. The entire study population was on aspirin therapy,
which suppresses platelet aggregation but not platelet adhesion.
The oral route of magnesium supplementation may not have produced
magnesium concentrations high enough to inhibit platelet
aggregation, as has been seen in IV magnesium infusion or bolus
therapy. Thus, without apparent impact on in vitro platelet
aggregation, oral magnesium may reduce PDT formation through its
antiplatelet adhesion effects.
This study was limited by its modest size and the relatively
low-risk status of the population, which may have attenuated the
potential benefits of oral magnesium treatment. Our results
suggest a need for further studies with larger numbers of
patients and, possibly, higher doses of magnesium. The ex vivo
experimental model of thrombus measurement is of undetermined
clinical relevance, but it provided a simple and reproducible way
to study how blood elements interact with thrombogenic
surfaces.
This trial implies that the story of magnesium in acute MI,
while marked by debate and paradoxical evidence, is far from
over. Oral magnesium—an even easier and more economical
treatment than IV magnesium—may hold the promise of added
protection against reinfarction and arrhythmias, even in the
current setting of nearly universal antithrombotic
pharmacotherapy.
Using Oral Magnesium
According to the American College of Cardiology/American Heart
Association Guidelines for the Management of Patients with Acute
Myocardial Infarction, the weight of evicence favors the use of
magnesium to correct documented deficits in magnesium and
potassium, especially in patients who were receiving diurectics
before the onset of infarction. In particular, patients
exhibiting episodes of torsades de pointes-type ventricular
tachycardia associated with a prolonged QT interval should
receive 1 to 2 g magnesium administered as an IV bolus over 5
minutes. The relatively limited gastrointestinal absorption of
magnesium makes oral prepartions inappropriate for acute
indications.
As for the use of oral magnesium in patients with CAD, acute
MI, or cardiovascular risk factors, no firm guidelines have been
developed. Clinical judgement must play a central role in
identifying at risk patients and implementing supplementation,
including choice of formulation and dosage. Long-term magnesium
supplementation may benefit various cardiovascular conditions,
particularly hypertension. In particular, patients receiving
short- or long-term therapy with thiazide or loop diuretics are
candidates for magnesium replacement.
The potential of oral magnesium for ameliorating CAD will
remain untapped until clinicians become aware that patients may
be magnesium-deficient even when serum magnesium levels are
normal. Checking serum magnesium levels for frank hypomagnesemia
is especially important for patients hospitalized with CAD.
Some magnesium authorities advocate a more aggressive approach
to correcting the widespread dietary shortfall in magnesium
levels. The first step in a population-based approach is to
encourage adequate dietary intake of magnesium-rich foods. Oral
supplementation is reccommended for individuals with chronic
deficiencies that are unlikely to be connected through dietary
modification and for health patients with a high risk profile for
CAD or a family history of premature CAD.
Oral supplementation of 500 mg/d elemental magnesium or less
is considered safe for most individuals with normal renal
runction. The most common adverse effect at doses higher than 600
to 700 mg/d is diarrhea. Before starting supplementation, it is
advisable to assess renal runction; throughout supplementation,
measurements of serum magnesium and potassium levels are
recommended. The ideal duration of supplementation is unknown,
but should last up to several months. Oral magnesium use may be
tapered or discontinued once serum levels remain normal over the
course of several weeks, or if diarrhea develops.
Suggested Reading
Seelig, MS, Elin RJ. Is there a place for magnesium in the
treatment of acute myocardial infarction? Am Heart J.
1996;132:471-477.
Shechter M, Hod H, Chouraqui P, Kaplinsky E, Rabinowitz B.
Magnesium therapy in acute myocardial infarction when patients
are not candidates for thrombolytic therapy. Am J
Cardiol. 1995;75:3321-323.
Shechter M, Kaplinsky E, Rabinowitz B. Review of clinical
evidence—is there a role for supplemental magnesium in
acute myocardial infarction in high-risk populations (patients
ineligible for thrombolysis and the elderly)? Coron Artery
Dis. 1996;7:352-358.
Shechter M, Kaplinsky E, Rabinowitz B. The rationale of
magnesium supplementation in acute myocardial infarction. A
review of the literature. Arch Intern Med.
1992;152:2189-2196.
Shechter M, Merz NB, Paul-Labrador M, et al. Oral magnesium
supplementation inhibits platelet-dependent thrombosis in
patients with coronary artery disease Am J Cardiol.
1999;84:152-156.
Q&A: Using Oral Mg in CAD
Q: Do you use oral magnesium supplementation in your
practice?
DR. SHECHTER: Yes, oral supplementation of
magnesium is prescribed in our clinic for selected
patients.
Q: How do you decide whether a patient with CAD or
cardiovascular risk factors should receive oral
magnesium?
DR. SHECHTER: Most patients with CAD are
magnesium deficient, especially elderly ones, those with CHF, and
those taking digoxin. Unless they also have renal failure, these
patients are candidates for oral magnesium.
Q: Which patients are most likely to benefit from oral
magnesium?
DR. SHECHTER: Patients most likely to benefit
include those taking diuretics, digitalis, or laxatives; those
with diabetes mellitus; and, as I said, the elderly, who
typically have a low dietary intake of magnesium. Several other
factors also increase susceptibality to deficiency among elderly
patients; reduced intestinal absorption and increased urinary
output of magnesium, a high rate of disorders that impair
absorption and renal function, and widespread use of
magnesium-wasting medications.
Q: Which formulations of magnesium supplementation are best
absorbed
DR. SHECHTER: Magnesium oxide, which was used in
our clinical trial, is excellent. Other formulations with good
overall absorption and bioavailabilty characteristics are
magnesium gluconate and magnesium citrate.
Q: Which formulation do you prefer?
DR. SHECHTER: We use magnesium oxide in 400 mg
tablets, each containing 241.3 g(10.86 mEq) of elemental
magnesium, given 2 to 3 times daily.
The above article is from the "The Magnesium Report",
August 1999. Blaine Pharmaceuticals is the manufacturer of
Mag-Ox 400 and Uro-Mag magnesium supplements.

Go
to Blaine Pharmaceuticals
|
This page was first uploaded to The Magnesium Web Site on May
11, 2004
http://www.mgwater.com/




