CMA JOURNAL/AUGUST 9, 1975/VOL. 113
Ischemic heart disease, water hardness and myocardial
magnesium
T.W. ANDERSON,* BM, B CH, PH D; L.C. NERI,† MD, DPH, M
SC; G.B. SCHREIBER,‡ MS HYG; F.D.F. TALBOT,§ PH D; A.
ZDROJEWSKI,¶ DIP AG SCI
Summary: In 54 cases of accidental death in cities
with water hardness of 60 parts per million (ppm) or less, the
mean myocardial magnesium concentration was 918 µg/g of dry
tissue. This was 7% lower than the corresponding figure of 982
µg/g among 29 cases of accidental death in cities with
water hardness of 300 ppm or more, and this difference was
significant (P < 0.01). There were no significant differences
between the cities with soft and hard water in the mean
myocardial concentrations of calcium, zinc, copper, chromium,
lead or cadmium.
These results are compatible with the belief that the
relatively high death rates in some soft-water areas may be due
to a suboptimal intake of magnesium, and that water-borne
magnesium exerts a protective effect on the residents of
hard-water areas.
Résumé: Dans 54 cas de mort accidentelle
survenue dans des villes où l’eau de boisson
contenait 60 parties de sels minéraux par million (ppm) ou
moms, la concentration moyenne de magnésium dans le
myocarde était de 918 µg/g de tissu sec. Cette
concentration était 7% plus faible que celle de 982
µg/g parmi 29 personnes mortes accidentellement dans des
villes où la teneur de l’eau potable était au
moms de 300 ppm. Cette difference était significative (P
< 0.01). Par contre, en ce qui concernait lea concentrations
moyennes de calcium, de zinc, de cuivre, de chrome, de p1omb ou
de cadmium dans le myocarde on ne notait guère de
différences significatives entre les villes a eau dure et
celles à eau douce.
Ces résultats corroborent la théorie que
les taux de mortalité relativement élevés
constatés dans certaines régions a eau douce
pourraient être attribués a une ingestion
suboptimale de magnésium et que le magnésium
contenu dans l’eau de boisson exerce un effet protecteur
sur les résidents des régions dont l’eau est
dure.
A number of investigators have reported an association between
the hardness ["Hardness" reflects the mineral content of water as
measured by its capacity to prevent the lathering of a standard
soap solution. It is conventionally expressed in terms of parts
per million (ppm) ( ≈ mg/l) of calcium carbonate
equivalent.] of municipal water supplies and local mortality
rates, particularly (but not exclusively) those ascribed to
ischemic heart disease (IHD).1, 2 In some areas the
association between higher IHD mortality and water hardness
appears to be due to a higher frequency of "sudden deaths" in the
communities with softer water.3-6
If there is indeed a causal relation between water composition
and IHD mortality, the factor responsible is presumably something
toxic in soft water or something beneficial in hard water. If the
latter, two of the most likely factors are calcium and magnesium,
since they are mainly responsible for the hardness of most water
supplies and have been shown to have some of the strongest
correlations with regional mortality patterns.2, 4, 7
In a previous study we therefore examined serum concentrations of
these two elements in residents of soft- and hard-water
communities in Ontario, but were unable to find any difference in
serum values and thus had no reason to believe that residents of
soft-water areas were suffering from an intake of either calcium
or magnesium that was less than optimal.8
However, it seemed possible that a comparison of heart-muscle
concentrations of these elements might be more rewarding.
Crawford and Crawford demonstrated some years ago that the walls
of the coronary arteries of young residents of a soft-water area
had relatively low concentrations of both calcium and magnesium,
although the higher IHD death rate in the soft-water area
appeared to be due to an increased susceptibility of the
myocardium to infarction rather than to a difference in the
severity of coronary artery disease.9 Furthermore, in
the case of magnesium at least, tissue concentration could well
be a more appropriate indicator of adequate intake than serum
concentration since magnesium is (unlike calcium) predominantly
an intracellular ion.
Other reasons for believing that magnesium may be the elusive
"water factor" are the following:
1. Highly refined Western diets may supply barely adequate
amounts of magnesium, so that the additional water-borne
magnesium could be critical.10, 11
2. Myocardial magnesium concentrations have been found to be
abnormally low in persons dying from myocardial infarction, even
in the uninfarcted parts of the myocardium, suggesting that a low
concentration may predispose to infarction or to a fatal outcome
thereof.12-15
3. One of the manifestations of magnesium deficiency in
experimental animals is an increased tendency to cardiac
arrhythmias16 — possibly analogous to the
increased frequency of sudden death reported in some soft-water
areas.3-5
We therefore investigated samples of myocardial tissue taken
during autopsies of local residents in two areas at the extremes
of the water-hardness range in Ontario for their concentrations
of seven elements. In addition, samples were taken from the
diaphragm (as an example of another continuously active muscle)
and the pectoralis major. All three muscle samples were then
analysed for magnesium and calcium, as well as for zinc, copper,
chromium, cadmium and lead.
The data were subsequently examined in three groups according
to the cause of death — IHD, accidents and all other
causes. Because our primary interest was in the tissue
electrolyte concentrations of "normal" residents in the two
areas, the accidental deaths were of particular importance, for
although we recognize that these cases are not a random sample of
the general population, they are the closest available
approximation to such a sample.
Method and material
This study, conducted over a 4-month period, was carried out
with the cooperation of pathologists in eight Ontario cities.
Five of these cities (North Bay, Pembroke, Sault Ste. Marie,
Sudbury and Thunder Bay) have municipal water supplies of less
than 60 ppm total hardness, while three (Brantford, Guelph and
Stratford) have water supplies with a hardness of more than 300
ppm.
The pathologists were asked to obtain samples approximately 3
x 3 cm in size from the apex [The apex of the heart was chosen as
the part least likely to be involved in a recent but inapparent
myocardial infarction.] of the heart, the muscular part of the
diaphragm and the midsection of the pectoralis major. These were
to be placed in separate plastic containers, labelled and
transferred promptly to a deepfreeze. Brief information on age,
sex, cause of death, duration of final illness and place of usual
residence was also requested for each subject, and this
information was subsequently augmented when necessary by
reference to death certificates, coroner’s reports and
hospital records.
Samples were obtained from 161 autopsies. The cause of death
was accident or suicide in 83 and acute IHD in 40, but loss or
spoilage reduced the number of samples from a potential maximum
of 123 to 122 (myocardium), 116 (diaphragm) and 117 (pectoralis).
The remaining 38 deaths were due to a variety of other causes and
the data from these cases will not be examined in detail in this
report.
Most of the accidental deaths were due to trauma (notably
motor vehicle accidents), while among the suicides approximately
half were due to trauma (gunshot wounds or hanging) and half were
"chemical" (carbon monoxide, drug overdose, etc.). (Note: Here,
as in some other parts of this report, the accident and suicide
cases are, for convenience. referred to simply as accidents.)
All muscle samples were analysed in a central laboratory under
the supervision of one of us (G.B.S.). Each specimen was stripped
of all visible fat, blood vessels and fibrous or necrotic tissue,
then dried at 110°C to constant weight in a Kjeldahl flask.
Wet washing was carried out in the same flask; 15 ml of a 2:1
mixture of nitric and perchloric acids was used. After standing
overnight the mixture was heated gradually to approximately
220°C until a clear solution was obtained. Analyses for
magnesium, zinc and copper were carried out using a Pye Unicam
SP9O atomic absorption spectrophotometer, with either an
air/acetylene flame (magnesium) or an air/propane flame (zinc and
copper). For magnesium determinations a 1% solution of lanthanum
chloride was added to overcome interference from other ions. For
determinations of the other metals a Perkin-Elmer 403 atomic
absorption spectrophotometer was used, with a nitrous
oxide/acetylene flame for calcium and a PE HGA-70 graphite
furnace for chromium, lead and cadmium. Quality control was
maintained by standard additions and by replicate analysis using
anode-stripping voltametry.
Concentrations were calculated in µg/g of both the wet
and dry weights of the tissue samples. Initial comparisons were
carried out using both wet and dry values and yielded identical
conclusions. For simplicity, only the dry-weight concentrations
will be given in this report. (Wet-weight concentrations were
approximately 25% of the dry values.) Extreme outlier values were
adjusted by a modification of the method suggested by Snedecor
and Cochran,17 so that each outlier was then plus or
minus two standard deviations from the mean. Three magnesium
values (two diaphragm, one pectoralis) and four calcium values
(three myocardium, one pectoralis) were thus treated. In each
case there was a slight effect on the mean, but the main effect
was to reduce the size of the standard error of the mean. In no
case did this manipulation change the interpretation of the
results.
Scatter diagrams of the data, subdivided by region, cause of
death and type of tissue, demonstrated approximately normal
distributions of magnesium, calcium, zinc and copper
concentrations, but many of the chromium and cadmium values were
close to zero, resulting in very skewed distributions. A log
transformation was therefore carried out on the chromium and
cadmium values before means and standard errors were calculated,
making the few zero values equal to 0.01 µg/g (the lower
limit of detection).
Results
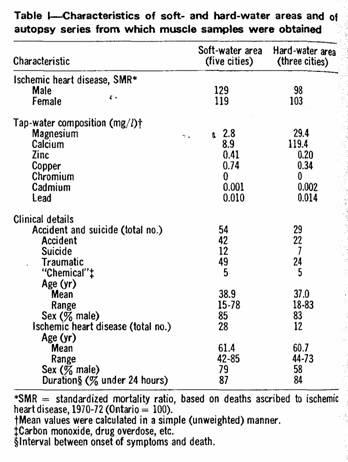
Some of the characteristics of the five soft-water and three
hard-water communities and of the autopsy material therefrom are
shown in Table I. Water analyses were based on samples from 4 to
20 household tap-water samples in each city.
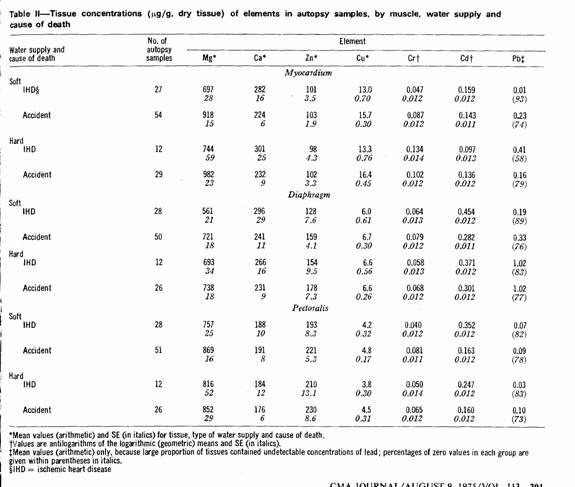
The results of the tissue analyses are summarized in Table II.
To facilitate comparisons of the mean concentrations by type of
water supply and by cause of death, certain ratios were
calculated; these are presented in Figs. 1 and 2. Lead values
were omitted because of the large proportion of zero
(undetectable) values.
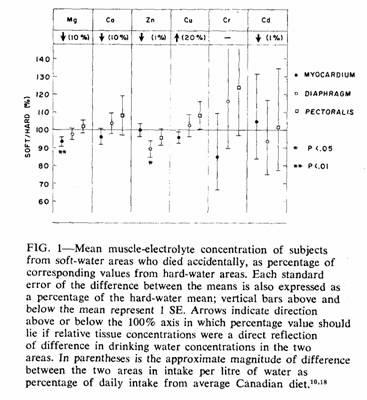
In Fig. 1 only accident cases are considered. Magnesium was
the only element with a significant difference in myocardial
concentration between the two areas (t = 2.43). Since
this difference was in the direction predicted by the hypothesis
und examination, a one-tailed t test was appropriate and
gave a P value of less than 0.01. To ensure that this finding was
not due to an abnormal distribution of values within a single
subgroup, the means were recalculated after classification
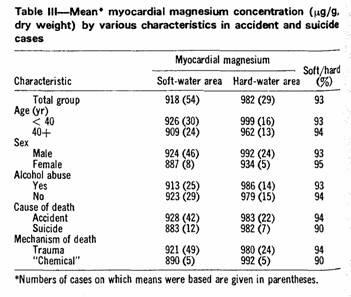
according to various characteristics (Table III). In each case
the direction and magnitude of the difference in myocardial
magnesium concentration were similar.
The only other significant difference between the accident
means was that for diaphragm zinc concentration (t =
2.44). Since there was no reason to anticipate this difference, a
two-tailed t test was appropriate and gave a P value
between 0.05 and 0.01.
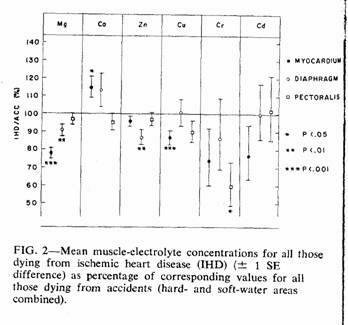
In Fig. 2 a comparison is made between IHD deaths and
accidental deaths, using the combined results from both
water-hardness areas. The accident means (forming the 100% axis)
were adjusted to allow for the more than 20-year difference in
mean age between the subjects who died accidentally and those who
died from IHD (Table I). Linear regressions on age found to be
significant (based on the total accident cases), together with
the sign of the relation (+ = direct correlation; - = inverse
correlation) were as follows: myocardium, Ca(+), Cu(-);
diaphragm, Mg(-), Cd(+); pectoralis, Mg(-), Zn(-), Cd(+).
Adjustment was carried out within each muscle subgroup by using
the appropriate regression coefficient to bring the mean
concentration for each subject who died accidentally to what it
would have been at the mean age of those who died from IHD, then
recalculating the mean value. In most of the subgroups this
adjustment reduced the size of the difference between the IHD and
accident means and in no case did it create a significant
difference where none had previously existed.
There were significant differences (both before and after
age-adjustment) in the myocardial concentrations of magnesium,
calcium and copper, the diaphragm concentrations of magnesium and
zinc, and the pectoralis concentrations of chromium.
Discussion
These results are compatible with the belief that the higher
cardiac death rate in the soft-water areas of Ontario is due to
the relative lack of magnesium in the water supply.
Among those who died accidentally the mean myocardial
magnesium concentration in the soft-water area was 93% of that in
the hard-water area (Fig. 1, Table II). It is unlikely that this
difference was due to chance. Not only was the difference
significant, but differences of similar direction and magnitude
were present in samples from both those who died from IHD (94%)
and those who died from "other causes" (88%). Data for the latter
group have not been examined in any detail in this paper because
in most cases there was a prolonged period of failing health
(e.g. cancer) or metabolic disturbance (e.g. diabetes) that might
have affected tissue electrolyte concentrations. However, for the
20 individuals who died from other causes in the soft-water area
the mean myocardial magnesium concentration was 828 µg/g,
some 88% of the corresponding value of 943 µg/g in the 18
such individuals in the hard-water area.
The fact that the diaphragm and pectoralis muscles did not
have the same sort of soft:hard differential in magnesium
concentrations as the myocardium would seem at first to argue
against the myocardial differential being due to a deficient
magnesium intake by the residents of the soft-water areas.
However, animal experiments have shown that a prolonged mild
restriction magnesium intake can depress the magnesium
concentration of the myocardium while having little effect on the
magnesium content of skeletal muscle.19
The regional difference in these results could have arisen as
a result of an imbalance between the two groups in the frequency
of other conditions associated with abnormally low magnesium
concentrations. Although a number of such conditions
exist,20 the only two that are common enough to pose a
problem in the present context are alcoholism and the prolonged
use of diuretics. We did not have a detailed clinical history for
each subject, but "normal" persons (i.e. those who died
accidentally) under the age of 40 are unlikely to have been
taking diuretics, and the regional difference in this age group
was actually slightly greater (93%) than in those aged 40 or more
(94%) (Table III). Acute or chronic alcohol abuse was recorded in
almost half of the accident cases, but the proportion was almost
identical in the two areas (soft, 46%; hard, 48%). Furthermore,
the regional difference in myocardial magnesium concentration was
essentially the same in those with and those without a history of
alcohol abuse (Table III). Greater use of alcohol or diuretics by
the individuals who had lived in soft-water areas can therefore
not explain their lower concentrations of myocardial
magnesium.
Since we carried out no dietary studies we are unable to rule
out the possibility that the residents of the soft-water areas
may consume a diet that contains less magnesium than the diet of
the hard-water-area residents, but this is unlikely since the
availability of different types of food is relatively uniform
throughout Ontario. In any case, if there is such a difference in
the dietary intake of magnesium it w1l be accentuated by the
difference in water-borne magnesium, particularly since the
latter is ionized and therefore likely to be more readily
available than the magnesium in food. Furthermore, if magnesium
deficiency is the cause of the higher cardiac mortality in the
soft-water area, prevention could presumably be achieved by oral
magnesium supplementation, whether the deficiency was originally
in the food-borne or water-borne supply.
The mean myocardial magnesium of those who died from IHD was
22% lower than the age-adjusted mean of those who died
accidentally (Fig. 2). This value was well within the 19 to 32%
range reported by other investigators in the "normal" (i.e.
uninfarcted) myocardium of persons dying from myocardial
infarction,7 and was highly significant. Those who
died from IHD were also characterized by a relatively low
magnesium concentration in both the diaphragm and pectoralis
muscles, a finding that is consistent with the hypothesis that
myocardial infarction may be in part the manifestation of a
generalized muscle disorder.21, 22
Calcium, the element that, together with magnesium, is
responsible for most of the hardness of Ontario water supplies,
also was found in a slightly lower myocardial concentration in
the subjects from the soft-water area. However, the difference
did not reach statistical significance (t = 0.7, P >
0.2) and, furthermore, death from IHD was associated with a
significantly higher concentration of myocardial calcium than
normal (Fig. 2) — a difference opposite to that which would
be expected if fatal IHD was associated with an inadequate
calcium intake. In view of the close interrelationship of calcium
and magnesium20, 23 it is possible that the elevated
calcium values in the IHD subjects were secondary to the low
magnesium values. This is also consistent with recent findings in
experimental animals.24
Zinc was the only metal other than magnesium with a
significant difference in concentration in soft- and hard-water
areas for those who died accidentally and, although this
difference was confined to the diaphragm samples, it was also
present (and significant) in the IHD:accident comparison. The
implications of these differences are not immediately obvious,
since not only were the myocardial zinc comparisons unremarkable,
but water supply makes only a limited contribution (approximately
1 or 2%) to total zinc intake.18
Myocardial copper concentrations were significantly lower in
those who died from IHD (Fig. 2); the soft:hard difference was in
the same direction but was not significant. However, the copper
content of drinking water was higher in the soft- than the
hard-water area (Fig. 2), probably because of a leaching effect
on the copper pipes used in the household water distribution
system.
Chromium is also unlikely to be the "water factor" since,
although myocardial concentrations showed differences that were
in the right direction, the differences were nonsignificant and
the metal was undetectable in the water supplies of either
area.
However, although zinc, copper and chromium do not appear
likely to be the water factor, they may contribute more to the
modern epidemic of IHD than is generally recognized. It is
possible, of course, that each of the low values from subjects
who died from IHD may be simply a secondary manifestation of a
diseased muscle, but some could equally well be attributable to a
dietary deficiency that, if rectified, might improve the health
of the muscle and reduce the vulnerability of the myocardium to
infarction.
Cadmium and lead are the only two metals measured in this
study that have no known useful biologic function. Both usually
tend to be higher in concentration in water from soft-water areas
(as a result of corrosion of metal pipes), although in the
present study concentrations were actually slightly higher in the
hard-water areas. Tissue concentrations of cadmium were not
convincingly different between hard- and soft-water areas or
between IHD and accident victims. Most of the muscle samples
contained no measurable amounts of lead (Table II), and there was
no evidence of any systematic differences in those samples that
did contain measurable amounts of lead. Neither lead nor cadmium
would therefore appear to contribute to the heart
disease—water correlations in Ontario, although it is
possible that they are important in other parts of the world.
We acknowledge with thanks the assistance of Dr. H.B. Cotnam,
supervising coroner of Ontario, Mr. H.F.C. Humphries, deputy
registrar general of Ontario; and Drs. EN. Alcantara, J.E.
Bazinet, RE. Boom, A.E. Croal, EM. Fernandez, L.A. Jentz, F.J.
Lone, K.F. Oliveira, BR. Oliver, J.L. Penistan (deceased), B.
Rasaiah, MA. Scarff, M.J.P. Schryer, F.P. Sparks, S.J. Strong,
R.G. Tasker and P. Wentworth. We are also grateful to D. Hewitt,
J.R. Marier, M.D. Silver and P.N. Corey for their very helpful
advice.
References
1. NERI LC, MANDEL JS, HEWITT D: Relation between mortality
and water hardness in Canada. Lancet 1: 931, 1972
2. NERI LC, HEWITT D, SCHREIBER GB: Can epidemiology elucidate
the water story? Am J Epidemiol 99: 75, 1974
3. ANDERSON TW, LE RICHE WH, MACKAY JS: Sudden death and
ischemic heart disease: correlation with hardness of local water
supply. N Engl J Med 280: 805, 1969
4. ANDERSON TW, LE RICHE WH: Sudden death from ischemic heart
disease in Ontario and its correlation with water hardness and
other factors. Can Med Assoc J 105: 155, 1971
5. PETERSON DR. THOMPSON DJ, NAM JM: Water hardness,
arteriosclerotic heart disease and sudden death. Am J
Epidemiol 92: 90, 1970
6. NERI LC, HEWITT D, MANDEL JS: Risk of sudden death in soft
water areas. Am J Epidemiol 94: 101, 1971
7. SEELIG MS, HEGGTVEIT HA: Magnesium interrelationships in
ischemic heart disease: a review. Am J Clin Nutr 27: 59,
1974
8. ANDERSON TW: Serum electrolytes and skeletal mineralization
in hard- and soft-water areas. Can Med Assoc J 107: 34,
1972
9. CRAWFORD T, CRAWFORD MD: Prevalence and pathological
changes of ischaemic heart-disease in a hard-water and in a
soft-water area. Lancet 1: 229, 1967
10. SEELIG MS: The requirement of magnesium by the normal
adult. Am. J. Clin Nutr 14: 342, 1964
11. HANKIN JH, MARGEN S, GOLDSMITH NF: Contribution of hard
Water to calcium and magnesium intakes of adults. J Am Diet
Assoc 56: 212, 1970
12. ISERI LT, AEXANDER LC, MCCAUGHEY RS, et al: Water and
electrolyte content of cardiac and skeletal muscle in heart
failure and myocardial infarction. Am Heart J 41: 215,
1952
13. HEGGTVEIT HA, TENSER P, HUNT B: Magnesium content of
normal and ischemic human heart, in Proceedings of the 7th
International Congress of Clinical Pathology,
Montréal, 1969, p 53
14. CHIPPERFIELD B, CHIPPERFIELD JR: Heart-muscle magnesium,
potassium, and zinc concentrations after sudden death from
heart-disease. Lancet 2: 293, 1973
15. BEHR G, BURTON P: Heart-muscle magnesium. Ibid, p 450
16. SELLER RH: The role of magnesium in digitalis toxicity.
Am Heart J 82: 551, 1971
17. SNEDECOR GW, COCHRAN WG: Statistical Methods, 6th
ed. Ames, Iowa, Iowa State U Pr, 1967, p 157
18. KIRKPATRICK DC, COFFIN DE: The trace metal content of
representative Canadian diets in 1970 and 1971. Can Inst Food
Sci Technol J 7: 56, 1974
19. HUNT BJ: Age and magnesium deficiency in the rat with
emphasis on bone and muscle magnesium, Am J Physiol 221:
1809, 1971
20. WACKER WEC, PARISI AF: Magnesium metabolism. N Engl J
Med 278: 712, 1968
21. ANDERSON TW: The changing pattern of ischemic heart
disease. Can Med Assoc J 108: 1500, 1973
22. Idem: Nutritional muscular dystrophy and human Myocardial
Infarction. Lancet 2: 298, 1973
23. MARIER JR, ROSE D, BELANGER LF: Hard waters and heart
disease (C). Br Med J 2: 686, 1963
24. SILVER BB, SORSAHL LA: Magnesium modulation of calcium
uptake by heart mitochondria. Physiologist 3: 452,
1973
*Professor, department of preventive medicine and
biostatistics, faculty 0 medicine, University of Toronto
†Bureau of epidemiology, laboratory centre for disease
control, Health and Welfare Canada, Ottawa
‡Planning and evaluation directorate, health protection
branch, Health and Welfare Canada
§Professor, department of chemical engineering, University
of Ottawa
¶ Chemistry division, air pollution control directorate.
Department of the Environment, Ottawa
This work was supported by the Ontario Ministry of Health
under grant PR 21.
Reprint requests to: Dr. T.W. Anderson, Department of
preventive medicine and biostatistics, Faculty of medicine,
University of Toronto, Toronto, Ont. M5S 1A8
This page was first uploaded to The Magnesium Web Site on
August 23, 2002
http://www.mgwater.com/





