The New England Journal of Medicine, June 23,
1994
Improved Mineral Balance and Skeletal Metabolism in
Postmenopausal Women Treated with Potassium Bicarbonate
ANTHONY SEBASTIAN, M.D., STEVEN T. HARRIS, M.D., JOAN H.
OTTAWAY, M.A., KAREN M. TODD, M.S., R.D., AND R. CURTIS MORRIS,
JR., M.D.
Abstract Background.
In normal subjects, a low level of metabolic acidosis and
positive acid balance (the production of more acid than is
excreted) are typically present and correlate in degree with the
amount of endogenous acid produced by the metabolism of foods in
ordinary diets abundant in protein. Over a lifetime, the
counteraction of retained endogenous acid by base mobilized from
the skeleton may contribute to the decrease in bone mass that
occurs normally with aging.
Methods. To test that possibility, we
administered potassium bicarbonate to 18 postmenopausal women who
were given a constant diet (652 mg [16 mmol] of calcium and 96 g
of protein per 60 kg of body weight). The potassium bicarbonate
was given orally for 18 days in doses (60 to 120 mmol per day)
that nearly completely neutralized the endogenous acid.
Results. During the administration of
potassium bicarbonate, the calcium and phosphorus balance became
less negative or more positive — that is, less was excreted
in comparison with the amount ingested (mean [±SD] change
in calcium balance, +56±76 mg [1.4±1.9 mmol] per
day per 60 kg; P = 0.009; change in phosphorus balance,
+47±64 mg [1.5±2.1 mmol] per day per 60 kg; P =
0.007) because of reductions in urinary calcium and phosphorus
excretion. The changes in calcium and phosphorus balance were
positively correlated (P <0.001). Serum osteocalcin
concentrations increased from 5.5±2.8 to 6.1 ±2.8
ng per milliliter (P <0.001), and urinary hydroxyproline
excretion decreased from 28.9±12.3 to 26.7±10.8 mg
per day (220±94 to 204±82 µmol per day; P =
0.05). Net renal acid excretion decreased from 70.9±10.1
to 12.8±21.8 mmol per day, indicating nearly complete
neutralization of endogenous acid.
Conclusions. In postmenopausal women, the
oral administration of potassium bicarbonate at a dose sufficient
to neutralize endogenous acid improves calcium and phosphorus
balance, reduces bone resorption, and increases the rate of bone
formation, (N Engl J Med 1994; 330:1776-81.)
The skeleton is a reservoir of labile calcium that is
responsive to humoral mechanisms that maintain the concentration
of ionic calcium in extracellular fluid within narrow limits. The
skeleton is also a reservoir of labile base (in the form of
alkaline salts of calcium) that can be mobilized for the defense
of blood pH and plasma bicarbonate concentrations. The role of
the skeleton in acid-base homeostasis in adults may contribute to
the progressive decline in bone mass that occurs with age, which
is ultimately expressed as osteoporosis.1 The bone
loss may result, at least partly, from lifelong mobilization of
skeletal calcium salts to balance endogenous acid generated from
dietary precursors.1
Two conditions must be present for the normal dietary acid
load to contribute to the age-related decline in bone mass: a
diet-dependent signal for mobilization of the skeletal-base
reserve must be continually present, and a fraction of the daily
load of endogenous acid must neutralize base from bone and
therefore not appear as acid excreted in the urine. Both
conditions have been confirmed experimentally. Regarding the
first, in subjects eating ordinary diets, blood pH and plasma
bicarbonate concentrations are reduced progressively as
endogenous acid production is increased within the normal
range.2,3 Reductions in extracellular pH and plasma
bicarbonate concentrations are potent and independent signals for
the stimulation of bone resorption and inhibition of bone
formation.4-7 With respect to the second, in healthy
subjects eating ordinary diets in whom the plasma acid-base
composition is stable, net renal acid excretion does not fully
account for endogenous acid production.2,8
We investigated whether the long-term reduction in the net
production of endogenous acid that results from the oral
administration of alkali can reduce bone loss. Before initiating
long-term studies, we studied postmenopausal women to determine
whether reducing the net production of endogenous acid by means
of the short-term administration of a particular alkali
(potassium bicarbonate for a few weeks) improved calcium and
phosphorus balance and reduced bone resorption or increased bone
formation.
METHODS
We carried out studies of calcium and phosphorus balance in 18
women who were hospitalized in the General Clinical Research
Center of Moffitt-Long Hospitals, San Francisco. The committee on
human research approved the protocol, and each woman gave
informed consent. The women were white and ranged in age from 51
to 77 years, in weight from 53 to 76 kg, in height from 153 to
175 cm, and in body-mass index (the weight in kilograms divided
by the square of the height in meters) from 21 to 28. All had
undergone menopause at least five years earlier, were physically
active, were taking no medications or hormones, and had normal
blood pressure; one was a vegetarian. All were within the
expected weight range for their height and frame size according
to the method of Weigley and Metropolitan Life Insurance tables
for 1983. The bone density of the lumbar spine, measured by
computed tomography in 16 women, averaged 94.6 mg per cubic
centimeter (range, 47.1 to 144.1). The women’s z scores,
defined as the number of standard deviations from the average
value in a larger group of normal women of the same age studied
in the same laboratory, averaged -0.27 (range, -1.6 to +2.1). The
bone density of the spine, measured by dual-energy x-ray
absorptiometry in nine women, averaged 0.78 mg per square
centimeter (range, 0.58 to 0.89), which yielded z scores
averaging -1.1 (range, -2.7 to +0.2). Four women had evidence of
vertebral compression fractures on radiography.
The women were given a constant daily diet containing the
following mean (±SD) amounts of nutrients per 60 kg of
body weight: calcium, 652± 180 mg (16±5 mmol);
phosphorus, 871±51 mg (28±2 mmol); potassium,
59±3 mmol; sodium, 119±3 mmol; protein, 96±1
g; and energy, 1995±17 kcal. To facilitate adaptation to
the diet, each woman’s customary calcium intake was taken
into consideration in determining her calcium intake (adjusted
with calcium carbonate) for the study, and each woman followed
the diet for 20 to 22 days before a 6-day control period. After
the control period, potassium bicarbonate (60 to 120 mmol per day
per 60 kg in aqueous solution) was provided as an alkali
supplement for 18 days then discontinued for a 12-day recovery
period, during which dietary intake was otherwise kept constant.
We chose potassium rather than sodium bicarbonate because
potassium citrate, but not sodium citrate, induced a reduction in
urinary calcium concentrations in men with uric acid
nephrolithiasis.10 We hypothesized that in
postmenopausal women, the administration of potassium bicarbonate
would improve the external mineral balance.
The control periods were six days in length, with pooled stool
samples marked with brilliant blue dye. The amount of potassium
bicarbonate in stool was determined from the recovery of an
ingested nonabsorbable marker (polyethylene glycol).
Sample Collection
Arterialized venous blood samples were collected between 3:30
p.m. and 4:30 p.m., at least three hours after the noon meal,
without stasis or exposure to air, from a vein on the back of the
hand, which was warmed in a water bath at 44°C for five
minutes. The frequency of sampling during each of the three study
periods (the control, supplementation, and recovery periods)
varied, depending on the variable. We analyzed the specimens for
blood pH and carbon dioxide tension; plasma total carbon dioxide,
sodium, potassium, and chloride levels; and serum creatinine,
total calcium, ionized calcium, magnesium, and inorganic
phosphorus levels (measured 4 times during the control period, 10
times during the supplementation period, and 7 times during the
recovery period); serum 25-hydroxyvitamin D levels (measured once
during each period); and serum 1,25-dihydroxyvitamin D,
parathyroid hormone, and osteocalcin levels (measured 4, 6, and 3
times).
Each voided urine specimen was divided in half; one half was
preserved in acid for measurement of calcium, and the other half
was maintained under a thin layer of mineral oil preserved with
thymol, and pooled in 24-hour collections for the determination
of pH and carbon dioxide content. In addition, we measured the
total volume and the concentrations of ammonium, titratable acid,
sodium, potassium, chloride, inorganic phosphorus, magnesium, and
creatinine in the 24-hour samples. Hydroxyproline was measured in
three, six, and four 24-hour samples from the three periods,
respectively.
Because the composition of the diet and the amounts of
nutrients ingested by each woman were constant throughout the
study, urinary hydroxyproline excretion was not affected by
variations in collagen intake; therefore, changes in
hydroxyproline excretion were interpreted as indicating changes
in the rate of bone resorption.11-14 Changes in the
serum osteocalcin concentration were considered to indicate
changes in the rate of bone formation.13,15-18
Dietary intake was determined from analyses of duplicate
diets.
Laboratory Methods
The methods used for measuring the acid-base mineral, and
electrolyte analytes have been described previously. Ionized
calcium was measured in heparinized whole blood with a Nova 8
ionized calcium-pH analyzer (Nova Biomedical, Newton, Mass.).
Serum osteocalcin and parathyroid hormone were measured by
radioimmunoassay and calcitriol by radioreceptor assay, with
assay kits obtained from Nichols Institute (San Juan Capistrano,
Calif.).
Statistical Analysis
The results were analyzed by repeated-measures analysis of
variance of the average values for the three study periods for
each woman and post hoc paired comparisons by the
Student-Newman-Keuls test, with SAS software. The results are
presented as means ±SD.
RESULTS
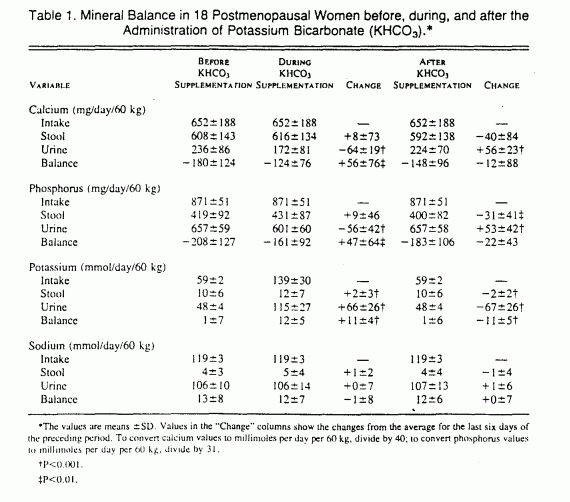
The calcium balance was negative — that is, more calcium
was excreted than ingested — throughout the study, but it
was significantly less negative during the period of
supplementation with potassium bicarbonate than during the
control period (P = 0.009) (Table 1). After the discontinuation
of potassium bicarbonate supplementation, calcium balance
returned toward the more negative values during the control
period. The net intestinal absorption of calcium was not
significantly influenced by the ingestion of potassium
bicarbonate. Rather, the potassium bicarbonate-induced
improvement in calcium balance was accounted for by a reduction
in urinary calcium excretion (Table 1 and Fig. 1).
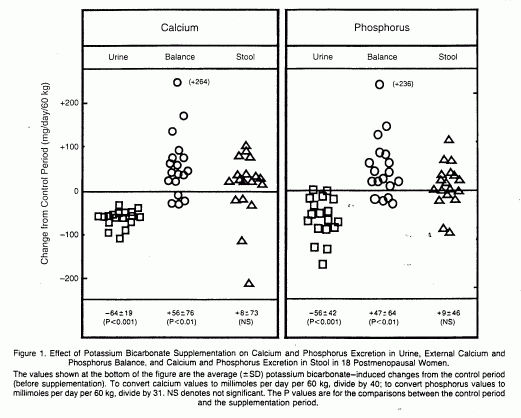
The findings with respect to the balance of inorganic
phosphorus were qualitatively similar to those for calcium (Table
1 and Fig. 1). The net intestinal absorption of phosphorus was
not significantly influenced by potassium bicarbonate
supplementation. On a molar basis, the potassium
bicarbonate-induced changes in calcium and phosphorus were
positively correlated (r=0.88, P<0.001).
Potassium balance was nearly zero (neutral) during the control
period, became positive during the period of potassium
bicarbonate supplementation, and returned to control-period
levels after potassium bicarbonate was discontinued (Table 1).
The sodium balance was slightly positive throughout the study
(Table 1).
Statistically significant and reversible increases in plasma
potassium and bicarbonate concentrations and blood pH occurred
during the administration of potassium bicarbonate (Table 2).
Neither the plasma ionized calcium concentration nor the total
inorganic phosphorus concentration changed significantly. The
serum 1,25-dihydroxyvitamin D concentration did not change during
the period of potassium bicarbonate supplementation, but it
increased significantly after supplementation was discontinued
(P<0.001). Serum parathyroid hormone concentrations increased
slightly but significantly (P = 0.019) during supplementation;
this increase persisted after potassium bicarbonate was
discontinued.
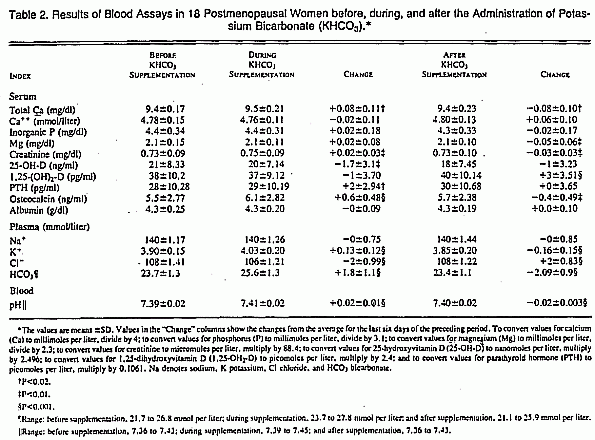
The mean serum osteocalcin concentration increased from
5.5±2.8 to 6.1±2.8 ng per milliliter (P<0.001)
(Table 2), and urinary hydroxyproline excretion decreased from
28.9±12.3 to 26.7±10.8 mg per day (220±94 to
204±82 µmol per day; P = 0.05) during potassium
bicarbonate administration.
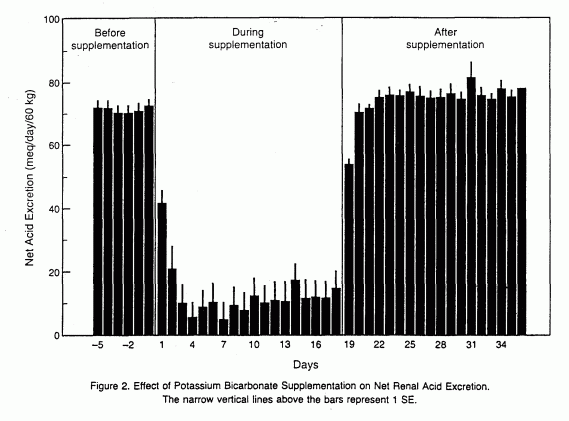
Net renal acid excretion decreased promptly toward zero after
the initiation of potassium bicarbonate supplementation (from
70.9± 10.1 mmol per day per 60 kg of body weight in the
control period to 12.8±21.8 mmol per day per 60 kg during
the supplementation period), indicating that endogenous acid was
almost completely neutralized during treatment (Fig. 2). After
potassium bicarbonate was discontinued, net acid excretion
returned promptly to control-period levels (73.2±9.9 meq
per day per 60 kg) (Fig. 2).
DISCUSSION
Bone mineral base is released into the systemic circulation
when exogenous acid is administered.3,4,20-22 When
acid loading is continued for several weeks or months, excretion
of acid in urine — quantitatively the principal component
of the homeostatic response to an exogenous acid load —
fails to keep pace with the increased load.20,21
Mobilization of bone alkali continues, bone mineral content and
bone mass progressively decline, and osteoporosis
occurs.23,24,26-29 In bone studied in vitro,
extracellular acidification increases the activity of
osteoclasts, the cells that mediate bone
resorption,7,30-32 and inhibits the activity of
osteoblasts, the cells that mediate bone
formation.7
Lifelong ingestion of ordinary diets constitutes a less
intense, more prolonged variant of the short-term experimental
administration of large exogenous acid loads. Typical American
diets are acid-producing in that renal excretion of acid exceeds
excretion of base, and when measured directly, the net balance of
endogenous acid (production less excretion) is
positive.3,8 The rate of endogenous acid production is
low, however, compared with that induced experimentally with
exogenous acid loads. On average, in healthy subjects eating
ordinary diets, net renal acid excretion very nearly equals
systemic net acid production, hence on average there is no
apparent retention of acid that might require, or induce,
mobilization of bone mineral base.3,8 But even with an
average value of zero for net acid balance, it is possible that
some people have a positive acid balance. Published data indicate
that a subgroup of healthy subjects do indeed retain acid in the
steady state.2 Acid balance correlates directly with
endogenous acid production.2 A person will have a
positive acid balance if his or her rate of acid production is in
the upper half of the normal range, and the balance will more
often be positive than negative when acid production is in the
mid-normal range.2
Thus, even with average rates of endogenous acid production,
the kidney fails to keep pace with acid production, with the
result that acid is continually retained in the body. Indeed, in
normal subjects eating diets that yield rates of endogenous acid
production spanning the normal range (0 to 150 mmol per day), we
found that steady-state blood acidity was higher and plasma
bicarbonate concentrations were lower in direct relation to the
steady-state rate of endogenous acid production.2
Clearly then, within its normal range, diet-dependent
production of endogenous acid can impose an acid load on the
body, resulting in both steady-state increases in blood acidity
and retention of acid.2 Although blood pH stabilizes
at a progressively lower level with each increasing level of acid
production within the normal range, the fact that stability
occurs at each level implies a continuing supply of base from an
internal reservoir, presumably the skeleton.
If typical acid-producing diets result in a continuing drain
on bone mineral base, supplementing the diets with exogenous base
might neutralize the acid produced and thereby eliminate the
drain on bone. In that case, calcium and phosphorus balance
should improve and the rate of bone resorption should decrease.
To test that possibility, we measured external calcium and
phosphorus balance and urinary hydroxyproline excretion in
postmenopausal women who received potassium bicarbonate (60 to
120 mmol per day per 60 kg) as a dietary supplement. The women
ate a typical whole-food diet and had a rate of production of
endogenous acid, estimated on the basis of their net acid
excretion (50 to 90 mmol per day per 60 kg), that predictably
produced a positive acid balance. The administration of potassium
bicarbonate induced a significant increase in both the calcium
and the phosphorus balance, resulting predominantly from the
reduced urinary excretion of these minerals. The improvement in
phosphorus balance correlated with an improvement in calcium
balance; on a molar basis, the slope of the relation was 1.0,
suggesting that one mole of phosphorus was retained per mole of
calcium retained. Since the phosphorus:calcium ratio in
hydroxyapatite is less than 1.0 (6:10), adequate phosphorus was
retained to permit calcium retention as hydroxyapatite.
Supplementation with potassium bicarbonate also reduced urinary
excretion of hydroxyproline, in association with an increase in
the serum osteocalcin concentration. Thus, the administration of
potassium bicarbonate appeared to reduce the rate of bone
resorption and increase the rate of bone formation.
Taken together, these results suggest that countering the
normal diet-related production of endogenous acid with orally
administered potassium bicarbonate can attenuate or reverse the
loss of bone mass that occurs over the long term in
postmenopausal women. Our findings extend those of
Lutz33 that the oral administration of alkali (by
means of substitution of sodium bicarbonate for dietary sodium
chloride) can improve calcium. balance in postmenopausal women,
those of Lemann et al.34 that orally administered
potassium bicarbonate (but not sodium bicarbonate) can improve
calcium and phosphorus balance in young men, and those of
Barzel35 that orally administered potassium
bicarbonate and sodium bicarbonate in combination can attenuate
the negative calcium balance induced by immobilization.
Increased plasma acidity or decreased plasma bicarbonate
concentrations might stimulate bone resorption directly by
favoring the physicochemical process of mineral dissolution and
indirectly by reducing the pH and bicarbonate concentration
within osteoclasts, thus promoting the adhesion of those cells to
their bone-resorptive sites and the secretion of hydrogen ions
into the subapical bone-resorbing fluid
compartment.4-7,26,30-32 Acidosis also inhibits
osteoblast function,7 potentially inhibiting bone
formation.
Our findings suggest that in postmenopausal women, dietary
supplementation with oral potassium bicarbonate in doses
sufficient to reduce the net production of endogenous acid
reduces the rate of bone resorption, increases the rate of bone
formation, and attenuates or reverses the loss of bone in defense
of systemic acid-base homeostasis. These findings are consistent
with current knowledge of the acid-base responses of osteoclasts
and osteoblasts studied in vitro; they suggest that the
age-related reduction in bone mass may result at least in part
from the cumulative effect of skeletal buffering of
diet-dependent endogenous acid production. The long-term
administration of potassium bicarbonate may therefore be
effective in preventing and treating postmenopausal
osteoporosis.
We are indebted to the staff of the General Clinical Research
Center, in particular the nursing staff under the direction of
DeAnna Sheeley, R.N., and the late Maureen Ford, R.N., and the
laboratory and dietary staff; to Ms. Patricia Douglass for her
administrative contributions at all phases of the project; to
Claude D. Arnaud, M.D., for counsel, encouragement, and support;
to Vicki McKee, F.N.P., for help in recruiting subjects and
implementing the protocol; to Anthony A. Portale, M.D., for
advice on laboratory methods and other helpful discussions; to
Mr. B. Muiz Brinkerhoff for data management; and to Renée
Merriam, RN., for organizational support.
REFERENCES
1. Wachman A, Bernstein DS. Diet and osteoporosis. Lancet
1968;l:958-9.
2. Kurtz I, Maher T, Hulter HN, Schambelan M, Sebastian A.
Effect of diet on plasma acid-base composition in normal humans.
Kidney Int 1983;24:670-80.
3. Kleinman JG, Lemann J Jr. Acid production. In: Maxwell MH,
Kleeeman CR, Narins RG, eds. Clinical disorders of fluid and
electrolyte metabolism. 4th ed. New York: McGraw-Hill.
1987;159-73.
4. Bushinsky DA. Internal exchanges of hydrogen ions: bone.
In: Seldin DW, Giebisch G. eds. The regulation of acid-base
balance. New York: Raven Press, 1989;69.88.
5.Bushinsky DA, Sessler NE, Krieger NS. Greater unidirectional
calcium efflux from bone during metabolic, compared with
respiratory, acidosis. Am J Physiol 1992;262:F425.F431.
6. Bushinsky DA, Sessler NE. Critical role of bicarbonate in
calcium release from bone. Am J Physiol 1992;263:F510-F515.
7. Krieger NS, Sessler NE, Bushinsky DA. Acidosis inhibits
osteoblastic and stimulates osteoclastic activity in vitro. Am J
Physiol 1992;262:F442-F448.
8. Lennon EJ, Lemann, J Jr, Litzow JR. The effects of diet and
stool composition on the net external acid balance of normal
subjects. J Clin Invest 1966;45:1601-7.
9. Weigley ES. Average? Ideal? Desirable? A brief overview of
height-weight tables in the United States. J Am Diet Assoc
1984;84:417-23.
10. Sakhace K, Nicar M, Hill K, Pak CYC. Contrasting effects
of potassium citrate and sodium citrate therapies on urinary
chemistries and crystallization of stone-forming salts. Kidney
Int 1983;24:348-52.
11. Epstein S. Serum and urinary markers of bone remodeling:
assessment of bone turnover. Endocr Rev 1988;9:437-49.
12. Deacon AC, Hulme P, Hesp R, Green JR, Tellez M, Reeve J.
Estimation of whole body bone resorption rate: a comparison of
urinary total hydroxyproline excretion with two radioisotopic
tracer methods in osteoporosis. Clin Chim Acta
1987;166:297-306.
13. Charles P, Poser JW, Mosekilde L, Jensen FT. Estimation of
bone turnover evaluated by 47Ca-kinetics: efficiency
of serum bone gamma-carboxyglutamic acid-containing protein,
serum alkaline phosphatase, and urinary hydroxyproline excretion.
J Clin Invest 1985;76:2254-8.
14. Klein L, Lafferty FW, Pearson OH, Curtiss PH Jr.
Correlation of urinary hydroxyproline, serum alkaline phosphatase
and skeletal calcium turnover. Metabolism 1964;13:272-84.
15. Hyldstrup L, Clemmensen I, Jensen BA, Transbøl I.
Non-invasive evaluation of bone formation: measurements of serum
alkaline phosphatase, whole body retention of diphosphonate and
serum osteocalcin in metabolic bone disorders and thyroid
disease. Scand J Clin Lab Invest 1988;48:611-9.
16. Johansen JS, Giwercman A, Hartwell D, et al. Serum bone
Gla-protein as a marker of bone growth in children and
adolescents: correlation with age, height, serum insulin-like
growth factor I, and serum testosterone. J Clin Endocrinol Metab
1988;67:273-8.
17. Worsfold M, Sharp CA. Davie MWJ. Serum osteocalcin and
other indices of bone formation: an 8-decade population study in
healthy men and women. Clin Chim Acta 1988;178:225-35.
18. Brown JP, Delmas PD, Malaval L, Edouard C, Chapuy MC,
Meunier PJ. Serum bone Gla-protein: a specific marker for bone
formation in postmenopausal osteoporosis. Lancet
1984;1:1091-3.
19. Jones JW, Sebastian A, Hulter HN, Schambelan M, Sutton JM,
Biglieri EG. Systemic and renal acid-base effects of chronic
dietary potassium depletion in humans. Kidney Int
1982;21:402-10.
20. Lemann J Jr, Litzow JR, Lennon EJ. The effects of chronic
acid loads in normal man: further evidence for the participation
of bone mineral in the defense against chronic metabolic
acidosis. J Clin Invest 1966;45:1608-14.
21. Lemann J Jr, Lennon EJ, Goodman AD, Litzow JR, Relman AS.
The net balance of acid in subjects given large loads of acid or
alkali. J Clin Invest 1965;44:507-17.
22. Lemann J Jr, Litzow JR, Lennon EJ. Studies of the
mechanism by which chronic metabolic acidosis augments urinary
calcium excretion in man. J Clin Invest 1967;46:1318-28.
23. Barzel US, Jowsey J. The effects of chronic acid and
alkali administration on bone turnover in adult rats. Clin Sci
1969;36:517-24.
24. Barzel US. The effect of excessive acid feeding on bone.
Calcif Tissue Res 1969;4:94-100
25. Burnell JM. Changes in bone sodium and carbonate in
metabolic acidosis and alkalosis in the dog. J Clin Invest
1971;50:327-31.
26. Delling G, Donath K. Morphometrische,
elektronenmikroskopische und physikalisch-chemische
Untersuchungen über die experimentelle Osteoporose bei
chronischer Acidose. Virchows Arch [A] Pathol Anat 1973;358:
321-30.
27. Barzel US. Acid-induced osteoporosis: an experimental
model of human osteoporosis. Calcif Tissue Res
1976;21:Suppl:417-22.
28. Jaffe HL, Bodansky A, Chandler JP. Ammonium chloride
decalcification, as modified by calcium intake: the relation
between generalized osteoporosis and ostitis fibrosa. J Exp Med
1932;56:823-34.
29. Bernstein DS, Wachman A, Hattner RS. Acid base balance in
metabolic bone disease. In: Barzel US, ed. Osteoporosis. New
York: Grune & Stratton. 1970;207-16.
30. Arnett TR, Dempster DW. Effect of pH on bone resorption by
rat osteoclasts in vitro. Endocrinology 1986;119:119-24.
31. Goldhaber P, Rabadjija L. H+ stimulation of
cell-mediated bone resorption in tissue culture. Am J Physiol
1987;253:E90-E98.
32. Teti A, Blair HC, Schlesinger P, et al. Extracellular
protons acidify osteoclasts, reduce cytosolic calcium, and
promote expression of cell-matrix attachment structures. J Clin
Invest 1989;84:773-80.
33. Lutz J. Calcium balance and acid-base status of women as
affected by increased protein intake and by sodium bicarbonate
ingestion. Am J Clin Nutr 1984;39:281-8.
34. Lemann J Jr, Gray RW, Pleuss JA. Potassium bicarbonate,
but not sodium bicarbonate, reduces urinary calcium excretion and
improves calcium balance in healthy men. Kidney Int
1989;35:688-95.
35. Barzel US. Alkali therapy in immobilization osteoporosis.
Israel J Med Sci 1971;7:499.
From the Department of Medicine and the General Clinical
Research Center, Moffitt-Long Hospitals, University of
California, San Francisco. Address reprint requests to Dr.
Sebastian at Box 0126, University of California, San Francisco,
CA 94143.
Supported by grants from the Public Health Service (P01DK
39964, R01 DK32631, and M01 00079) and the University of
California, San Francisco, Research Evaluation and Allocation
Committee (MSC-22) and Academic Senate, and by gifts from Church
and Dwight Company and the Emil Mosbacher, Jr., Foundation.
This page was first uploaded to The Magnesium Web Site on
December 18, 2002
http://www.mgwater.com/




