Human Physiology from Cells to Systems, Third Edition,
Lauralee Sherwood, Department of Physiology, School of Medicine,
West Virginia University, 530-531.
Fluid and Acid-Base Balance (Excerpt from Chapter 15.)
Fluctuations in hydrogen-ion concentration have
profound effects on body chemistry
Only a narrow pH range is compatible with life because even
small changes in [H+] have dramatic effects on normal
cell function. The prominent consequences of fluctuations in
[H+] include the following:
-
Changes in excitability of nerve and muscle cells arc
among the major clinical manifestations of pH abnormalities.
The major clinical effect of increased [H+]
(acidosis) is depression of the central nervous system.
Acidotic patients become disoriented and, in more severe
cases, eventually die in a state of coma, in contrast, the
major clinical effect of decreased [H+]
(alkalosis) is overexcitability of the nervous system, first
the peripheral nervous system and later the central nervous
system. Peripheral nerves become so excitable that they fire
even in the absence of normal stimuli. Such overexcitability
of the afferent (sensory) nerves give rise to abnormal
“pins and needles” tingling sensations. On the
other hand, overexcitability of efferent (motor) nerves
brings about muscle twitches and, in more pronounced cases,
severe muscle spasms. Death may occur in extreme alkalosis as
spasm of the respiratory muscles seriously impairs breathing.
Alternatively, severely alkalotic patients may die of
convulsions resulting from overexcitability of the central
nervous system. In less serious situations, CNS
overexcitability is manifested as extreme nervousness.
-
Hydrogen-ion concentration exerts a marked influence on
enzyme activity. Even slight deviations in [H+]
alter the shape and activity of protein molecules. Since
enzymes are proteins, a shift in the body acid-base balance
disturbs the normal pattern of metabolic activity catalyzed
by these enzymes. Some cellular chemical reactions are
accelerated; others are depressed.
-
Changes in [H+] influence K+ levels
in the body. When reabsorbing Na+ from the
filtrate, the renal tubular cells secrete either
K+ or H+ in exchange. Normally they
secrete a preponderance of K+ compared to
H+ Because of the intimate relationship between
secretion of H+ and K+ by the kidney an
increased rate of secretion of one of these ions is
accompanied by a decreased rate of secretion of the other.
For example, if more H+ than normal is eliminated
by the kidneys, as occurs when the body fluids become
acidotic, less K+ than usual can be excreted. The
resultant K+ retention can affect cardiac
function, among other detrimental consequences.
Hydrogen ions are continually being added to the body
fluids as a result of metabolic activities.
As with any other constituent, to maintain a
constant.[H+] in the body fluids, input of hydrogen
ions must be balanced by an equal output. On the input side only
a small amount of acid capable of dissociating to release
H+ is taken in with food, such as the weak citric acid
found in oranges Most H+ in the body fluids is
generated internally from metabolic activities. Normally
H+ is continually being added to the body fluids from
the three following sources:
1. Carbonic acid formation. The major source of
H+ is through H2CO3. formation
from metabolically produced CO2. Cellular oxidation
of nutrients yields energy, with CO2 and
H2O as end products. Catalyzed by the enzyme
carbonic anhydrase (ca), CO2 and
H2O form H2CO3 which then
partially dissociates to liberate free H+ and
HCO3-:
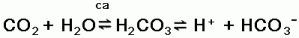
This reaction is reversible because it can proceed in either
direction, depending on the concentrations of the substances
involved as dictated by the law of mass action. Within
the systemic capillaries, the CO2 level in the blood
increases as metabolically produced CO2 enters from
the tissues. This drives the reaction to the acid side,
generating H+ as well as HCO3-
in the process. In the lungs, the reaction is reversed:
CO2 diffuses from the blood flowing through the
pulmonary capillaries into the alveoli (air sacs), from which
it is expired to the atmosphere. The resultant reduction in
CO2 in the blood drives the reaction toward the
CO2 side. Hydrogen ion and
HCO3- form H2CO3,
which rapidly decomposes into CO2 and H2O
once again. The CO2. is exhaled while the hydrogen
ions generated at the tissue level are incorporated into
H2O molecules.
When the respiratory system is able to keep pace with the
rate of metabolism, there is no net gain or loss of
H+ in the body fluids from metabolically produced
CO2. When the rate of CO2 removal by the
lungs does not match the rate of CO2 production at
the tissue level, however, the resultant accumulation or
deficit of CO2 in the body leads to an excess or
shortage, respectively, of free H+ in the body
fluids.
2. Inorganic acids produced during the breakdown of
nutrients. Dietary proteins and other ingested nutrient
molecules that are found abundantly in meat contain a large
quantity of sulfur and phosphorus. When these molecules are
broken down, sulfuric acid and phosphoric acid are produced as
by-products. Being moderately strong acids, these two inorganic
acids dissociate to a large extent, liberating free
H+ into the body fluids. In contrast, the breakdown
of fruits and vegetables produces bases that, to some extent,
neutralize the acids derived from protein metabolism.
Generally, however, more acids than bases are produced during
the breakdown of ingested food, leading to an excess of these
acids.
3. Organic acids resulting from intermediary
metabolism. Numerous organic acids are produced during
normal intermediary metabolism. For example, fatty acids are
produced during fat metabolism, and lactic acid is produced by
muscles during heavy exercise. These acids partially dissociate
to yield free H+.
Hydrogen-ion generation therefore normally goes on
continuously as a result of ongoing metabolic activities.
Furthermore, in certain disease states, additional acids may be
produced that further contribute to the total body pool of
H+. For example, in diabetes mellitus, large
quantities of keto acids may he produced as a result of abnormal
fat metabolism. Some types of acid-producing medications may also
add to the total load of H+ that must be handled by
the body. Thus, input of H+ is unceasing, highly
variable, and essentially unregulated.
The crux of H+ balance is maintaining the normal
alkalinity of the ECF (pH 7.4) despite this constant onslaught of
acid. The generated free H+ must be largely removed
from solution while in the body and ultimately must be eliminated
from the body so that the pH of the body fluids can remain within
the narrow range compatible with life. Mechanisms must also exist
to compensate rapidly for the occasional situation in which the
ECF becomes too alkaline.
Three lines of defense against changes in [H+]
operate to maintain the [H+] of body fluids at a
nearly constant level despite unregulated input: (1) the
chemical buffer systems, (2) the respiratory
mechanism of pH control, and (3) the renal mechanism of
pH control. We will look at each of these methods.
This page was first uploaded to The Magnesium Web Site on
December 19, 2002
http://www.mgwater.com/
