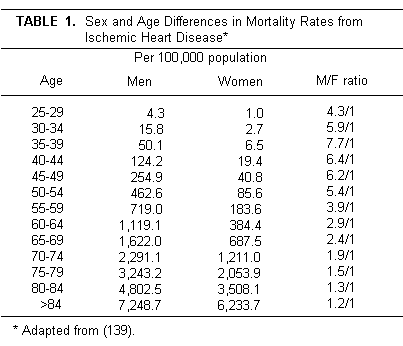In Journal of the American College of Nutrition, Vol. 12, NO.
4, 442-458 (1993)
INTERRELATIONSHIP OF MAGNESIUM AND ESTROGEN IN CARDIOVASCULAR
AND BONE DISORDERS, ECLAMPSIA, AND PREMENSTRUAL SYNDROME
Mildred S. Seelig, M.D., M.P.H., F.A.C.N.,
Adjunct Professor: Community and Preventive Medicine, New
York Medical College, Valhalla, New York, and Nutrition, School
of Public Health, University of North Carolina, Chapel Hill
The section headers of this paper are as follows:
The anticonvulsive and antihypertensive values of
magnesium (Mg) in eclampsia, and its antiarrhythmic applications
in a variety of cardiac diseases, have caused Mg to be considered
only for parenteral administration by many physicians. In
contrast, nutritionists have long recognized Mg as an essential
nutrient, because severe deficiencies elicit neuromuscular
manifestations similar to those justifying its use in eclampsia.
More recently, this element has been used to favorably influence
latent tetany with and without thrombotic complications, to delay
preterm birth, to influence premenstrual syndrome, and to
ameliorate migraine headaches. Most of these disorders
exclusively or largely afflict women. The lesions of arteries and
heart caused by experimental Mg deficiency have been well
documented and may contribute to human cardiovascular disease.
Estrogen's enhancement of Mg utilization and uptake by soft
tissues and bone may explain resistance of young women to heart
disease and osteoporosis, as well as increased prevalence of
these diseases when estrogen secretion ceases. However,
estrogen-induced shifts of Mg can be deleterious when estrogen
levels are high and Mg intake is suboptimal. The resultant
lowering of blood Mg can increase the CA/Mg ratio, thus favoring
coagulation. With Ca supplementation in the face of commonly low
Mg intake, risk of thrombosis increases.
Abbreviations:
AMI=acute myocardial infarction Ca=calcium cAMP-cyclic
adenosine monophosphate DM=diabetes mellitus EDRF=endothelial
derived relaxing factor HDL=high density lipoprotein
IHD=ischemic heart disease i.v.=intravenous IUGR=intrauterine
growth retardation LDL=low density lipoprotein MAP=mean
arterial pressure Mg=magnesium mmol=millimole OCA=oral
contraceptive agent PGI=prostacyclin potassium=K
PMS=premenstrual syndrome PTH=parathyroid hormone
RDA=recommended dietary allowances TBX=thromboxane VLDL=very
low density lipoprotein
In physiologic amounts, estrogen protects against
cardiovascular and bone diseases. In excess, especially in those
with marginal or deficient magnesium (Mg) intake, estrogen may
create problems. The observation that young women retaining Mg
better than do young men has led to speculation that this might
contribute to the sex difference in prevalence of ischemic heart
disease (IHD). Lower dietary Mg intake in the West compared to
the East may contribute to the relatively higher prevalence of
IHD in Western populations [1]. Experimental and epidemiologic
evidence implicating Mg deficiency in arteriosclerosis and IHD
has been reviewed elsewhere [2-8]. The much greater prevalence of
IHD in postmenopausal compared to young women, approaching that
of men, reflects the protective effect of estrogen. The benefits
of estrogen may be mediated in part by its effects on Mg
distribution [9-11]. Estrogen replacement therapy during
menopause is usually accompanied by increased intakes of calcium
(Ca). Rarely considered is fact that Mg increases bone elasticity
through formation and maintenance of bone matrix [5,9,12].
Development of osteoporosis in conditions of Mg malabsorption or
urinary wasting provides evidence that Mg deficiency can be
contributory to this disease [9].
Estrogen is deleterious at high levels. Thromboembolic events
have complicated synthetic estrogen treatment of prostatic
carcinoma [13], use as an oral contraceptive agent (OCA)
[9,14,15] and use to inhibit lactation [9,16,17]. High estrogen
secretion has been implicated in complications of pregnancy, for
which parenteral Mg is effective and protective [18-34].
Correction of underlying Mg deficiency, and pharmacologic effects
of Mg contribute to usefulness of Mg in cardiovascular disease
and eclampsia. Mg deficiency is associated with thrombogenesis
and arterioconstriction, which are pathogenic and complicating
factors in both conditions. At pharmacologic levels, the
anti-arrhythmic effects, formation of antiplatelet-aggregating,
vasodilating factors: prostacycline (PGI) and endothelial derived
relaxing factor (EDRF), and inhibition of formation of
platelet-aggregating, vasoconstricting factors: thromboxane (TXB)
and endothelin, were cited as justification of its use in a large
study to test Mg's efficacy in reducing mortality in acute
myocardial infarction (AMI) [35] . When estrogen is used to
prevent osteoporosis, and Mg intake is marginal or low, high
dosage Ca, which increases Mg needs [2-6], can keep the
pro-coagulative effects of Ca from being countered by the normal
anticoagulative effect of Mg in the circulation [5,9].
Preeclampsia, Eclampsia and Preterm
Labor
Treating toxemic pregnancy with intravenous (i.v.) Mg was
reported in 1925 [36]. In 1932, i.v. Mg was found to control
bovine hypocalcemic postpartum convulsions, to correct arrhythmia
caused by i.v.Ca, and to prevent or cure neuromuscular
irritability terminating in convulsions of lactating cows
consuming forage poor in Mg and rich in potassium (K) [37].
Diuretics and anti-convulsants displaced Mg for a time, in
treatment of eclampsia, until it was shown, in 1965, that Mg
treatment achieved better fetal salvage than did the new drugs
[38]. Accordingly, the position of parenteral Mg as treatment of
choice, has endured [39]. Mg is used now, as adjunct to tocolytic
therapy, or alone, to prevent premature uterine contractions and
preterm births (40-49).
Hypomagnesemia and Coagulopathy
Increased blood coagulability causes complications of toxemic
pregnancies: embolic events, phlebothrombosis, and
microthrombosis of glomeruli, and placental scarring that
interferes with fetal blood supply, thus resulting in
intrauterine growth retardation [IUGR]) [5,9,50-53]. Pulmonary
embolism may cause death during pregnancy and postpartum [51],
which suggests that naturally high estrogen secretion increases
intravascular coagulation. The latter may be mediated by
estrogen-induced shift of Mg from the blood into cells in the
face of marginal or low intake [9-11]. Hypomagnesemic pregnant
ewes had three-fold more platelets clumping than did normal ewes
[27]. In another study [54], hypomagnesemic cows and ewes
exhibited abnormal platelet activation. It has been postulated
that Mg deficiency in pregnant women increases coagulability.
Therefore use of Mg in eclampsia may reduce fetal and infant
complications [22,27,54].
Prostanoids
The production of glomerular microthrombi and lowered platelet
counts in pregnant Mg deficient ewes developing preeclampsia
called attention to the lowered ratio of PGI (vasodilator and
anti-platelet-aggregating prostanoid) to thromboxane
(vasoconstrictive, platelet-aggregating prostanoid) [27], that is
seen in Mg deficiency [27,55,56, infra vide] and implicated in
toxemias of pregnancy [57]. Furthermore, Mg increases formation
of PGI by human umbilical endothelial cells [58] and cord vessel
preparations [59]; endothelial cells from eclamptic patients who
had received Mg treatment produced 2-5 times more PGI than did
those not so treated [58]. Another potent circulating, naturally
occurring vasoconstrictor (endothelin), produced by vascular
endothelium, is more elevated in preeclamptic women before than
after Mg treatment. Umbilical venous endothelin of toxemic
patients was 10 times higher than in normal pregnant women [60].
Thus, not only has the use of Mg in eclampsia been scientifically
justified, there is now laboratory evidence that Mg deficiency
enhances intravascular blood coagulation and increases
hypertension, both directly and indirectly via resultant increase
in TXB and endothelin and decreases in EDRF and PGI.
Prophylaxis for Preeclampsia, Eclampsia and Preterm
Birth
Several investigators have suggested that low Mg intake and
tissue stores may contribute to eclampsia [18-34,41-46,61]. Mg
supplements have prevented premature uterine contractions and
reduced occurrence of calf cramps and sensations of numbness
[25,30,42]. A double-blind study disclosed fewer complications in
normal pregnant women given supplemental Mg than in controls
[29]. Mg deficiency, alone, caused preeclamptic hypertension in
pregnant triplet-bearing ewes [27]. Mg supplementation during
pregnancy also was not only associated with fewer preterm
deliveries, but fewer cases of IUGR [23,25,29-31,34,44,61]. In
Hungary, where the prevalence of preterm deliveries is very high,
a significant correlation was discerned between preterm delivery
rates and Mg concentration in drinking water [62]. Two groups of
pregnant women were randomly selected for a double blind study
concerning prophylactic Mg supplementation [63]. Among 255
expectant mothers who received 300 mg/day from diagnosis of
pregnancy to delivery, the preterm birth rate was 8.5%.; among
280 mothers given placebo but similarly managed prenatally, the
preterm birth rate was 10.9%, a significant difference. There
were too few spontaneous abortions to statistically evaluate the
difference observed (1.6% in Mg-group; 3.6% in controls).
However, supporting the premise that low Mg can contribute to
spontaneous abortion was the observation from Italy that
significantly lower Mg levels (and higher Ca/Mg ratios) were
found in women whose pregnancies had aborted than in those whose
pregnancies came to term [64]. Although a study in the United
States has not confirmed the protection afforded by Mg against
adverse outcomes of pregnancy [65]. routine Mg supplementation of
pregnant women, is increasingly being recommended overseas
[23,25,29-31,34,44,61].
With the foregoing data that support the premise that the
efficacy of high dose Mg treatment in complications of pregnancy
might be explained by repair of gestational Mg deficiency, as
well as by its pharmacologic activity, what evidence is there
that pregnant women are likely to be Mg deficient?
Metabolic Balance Evidence
We must rely principally on the early long-term metabolic
balance studies to estimate the amount of Mg required by pregnant
women for formation of new maternal and fetal tissue [66-69].
These extensive studies suggested that at least 450 mg/day was
required to maintain the strong positive balance needed for
maternal and fetal health. Of particular interest are data from a
multipara who had had repeated uncomplicated pregnancies and
healthy infants [68]. Intakes as high as 600 mg/day during the
last half of her pregnancy resulted in cumulative retentions of
15.5 grams. Daily Mg intakes below 300 mg resulted in negative
balance or bare equilibrium in an adolescent primipara [69]. Only
two Mg balance studies of pregnant women have been found, since
the studies of the 1930s [66-69]. One, a study of Ca requirements
during pregnancy, showed that three teen-age primiparas, on Ca
intakes of 2 g/day, had Mg balances of -14, +1 and +24, with mean
urinary Mg output of 124 mg/day in a six day balance period [70].
The only long-term metabolic balance study during pregnancy, to
provide data on Mg, was conducted in 10 healthy pregnant white,
middle class women [71]. With a mean Mg intake of 269 +/- 55 mg,
they had positive Mg balance in only 3 of 47 balance periods.
Their mean Mg balance was -40 +/- 50 mg; their mean urinary loss
was 94 +/- 28 mg, reflecting failure of renal conservation.
Renal Magnesium Excretion Studies
To examine whether inadequate Mg intake is related to toxemia
of pregnancy [72], the urinary Mg output (in mEq/g creatinine)
from 117 white, middle class women (aged 14-39 yrs, 10-42 weeks
gestation) was compared with their mean arterial pressure (MAP: 2
x diastolic pressure + systolic pressure divided by 3; normal =
about 90). There was significant negative correlation between MAP
and Mg excretion when it was less than 7.0 mEq/g creatinine, both
in 25 multiparous mothers and 32 primiparas. Four women had
values less than 2.0 mEq/g creatinine; all had hypertension, with
MAP > 105. In this study, those with the poorest Mg status had
the highest blood pressure. Percentage retention of Mg loads, by
measuring urinary Mg before and 24 hours after a parenteral Mg
load, is a practical means of determining Mg status; retention of
>20-25% has long been accepted as an index of Mg deficiency
[5,73]. Three studies have employed this test to demonstrate Mg
deficiency in normal pregnancies, and in those complicated by
hypertension or diabetes [21,74,75].
Dietary Surveys and Estimates
Dietary surveys of American pregnant women show that their Mg
intakes generally fall far short of needs. A 1976 report of the
Mg intake of pregnant women, estimated from food record diaries
from the fifth month of pregnancy until delivery [76] showed the
average intake to be 204 mg +- 54 (S.D.) mg/d (103 to 333 mg).
Almost 80% consumed less than 248 mg Mg in their normal diets.
There was a positive correlation between Mg intake and
birthweight of infants. Dietary estimates from 24-hour recall of
low income pregnant women indicated Mg intakes of 102, and 124
mg/1000 kcal for adolescent and older women; 97.3 and 92.1% of
the younger and older women, respectively, had Mg intakes less
than 100% of the 1980 RDA [77]. A 1987 review of mean dietary Mg
of pregnant women showed intakes to be 35-58% of the amount
deemed desirable: 450 mg/day [78]. Low income women consumed
97-100 mg Mg/1000 kcal; higher income women consumed an average
of 120 mg Mg/1000 kcal, not taking into account the Mg in
drinking water. A 1991 report of 212 pregnant women and their
infants showed that maternal Mg intake was inversely related to
six month infant systolic pressure [79].
Recommended Dietary Allowances for Magnesium
The 9th edition of the RDA [80] lists 450 mg/day as the
requirement of pregnant women, i.e. 150 mg/day is added to the
amount recommended for non-pregnant women. The current edition
[81] lowered the base daily requirement to 4.5 mg/kg/day,
discounting the extensive metabolic studies showing negative Mg
balances during most periods on intakes of less than 5 mg/kg/day
[1,82]. This also ignores a more recent study of young women on
controlled diets providing 265-305 mg/day of Mg (4-5 mg/kg/day),
who remained in strong negative Mg balance over three consecutive
20-day balance periods [83]. The current RDA of only 40 mg of Mg
more per day during pregnancy than the amount for girls 15-18
(300 mg) or those 19-24 (280 mg) is inappropriate and possibly
dangerous. This is especially true for pregnant adolescents,
whose requirements for growth and development may compete with
those of the fetus. The current RDA for Mg was based in part on
the erroneous assumption that half the ingested Mg is absorbed
and that renal conservation can prevent deficiency, a conclusion
not substantiated by the metabolic studies. Accordingly, the
increased need of Mg for anabolic processes of gestation seems to
justify prophylactic Mg administration to pregnant women to lower
the risk of maternal and infant complications, while awaiting
results of further investigation. [84,85].
The interrelationship between Mg and estrogen may be the basis
for a strong association of migraine and eclampsia [86-88].
Migraine has been found to occur far more frequently in women who
have had preeclampsia than in those who have not, and 2.5 times
as often in patients with preeclampsia before the 34th week of
pregnancy, than in those with normal pregnancy [88]. Favorable
results have been reported in 80% of 3,000 women, given 200
mg/day of Mg for prophylaxis of eclampsia and migraine, when they
were taking OCA or were pregnant [56,87]. The higher incidence of
migraine among those prone to hypomagnesemia [25,89] and the
influence of Mg on prostanoids and thrombogenesis, support the
premise that Mg deficiency is involved in the pathogenesis of
migraine [56,87,89].
Additional factors that may be involved in the role played by
Mg deficiency in migraine relate, not only to prostaglandin
metabolism [90], but to serotonin release and vascular reactivity
to serotonin. In vitro studies have shown that platelets from
migraine sufferers release more serotonin than normal, which may
contribute to cerebral vasospasms [91]. Spasms of canine cerebral
arteries induced by serotonin have been reduced by Mg [92].
Release of serotonin from platelets is enhanced by Ca and
inhibited by Mg [93]. Serotonin levels are increased in Mg
deficient animals [24,94]. Ca-channel blockers are effective in
prophylaxis of migraine [95], and Mg is a physiologic Ca-channel
blocker [96]. The latter further supports Mg treatment for
migraine [97].
Mg deficiency has been associated with the premenstrual
syndrome (PMS) alone [98-101], or in combination with
inadequacies of zinc, linoleic acid and B vitamin (predominantly
pyridoxine) [102-104] and high Ca intake [103]. The condition has
been reported to respond to Mg supplements alone [101,104,105] or
in combination with trace minerals and vitamins [106,107].
Mechanisms that may explain the explain the development of PMS,
and its response to nutritional therapy, entail interrelations of
Mg with estrogen and B vitamins in prostanoid, catecholamine, and
serotonin synthesis and release [102].
Arrhythmia
Disturbances in cardiac rhythm caused by Mg deficiency were
first described in rats in 1932 and 1938 [108, 109]. The nature
of electrocardiogram changes caused by severe acute Mg deficiency
was related to K deficiency in rats [110] and dogs [111], and
then extended to longer-term subacute Mg deficiencies in dogs and
monkeys [112-115]. Additional details of experimental evidence of
the nature of Mg deficiency-induced dysrhythmias in cows and in
humans have been presented elsewhere [3,5,8]. Additional evidence
of Mg deficiency induced dysrhythmias in cows and humans has been
presented elsewhere [3,5,8]. In addition, the therapeutic
efficacy of i.v Mg was reported in cows with complications from
i.v. Ca treatment) [37]. In the 1930 arrhythmias were also
recognized as a risk of rapid i.v. Ca injections, used as an
adjunct to digitalis treatment or to measure circulation time
[116]. In contrast, i.v. Mg was recommended for circulation time
determinations as it was relatively free of deleterious effect on
the heart [117].
The efficacy of i.v Mg in controlling a variety of arrhythmias
was shown in both men and women in 1935 [118], but received
little attention until the 1975 evaluation of the role of Mg
deficiency and replacement in cardiac rhythmicity [119]. The
antiarrhythmic activity of Mg has since received widespread
attention. Cited here are only a few key papers that consider
prior Mg depletion as a pathogenic factor [120-123]. Use of Mg in
victims of acute myocardial infarction (AMI), has been clearly
demonstrated worldwide to improve survival [124-138]. The
anti-arrhythmic effect of Mg is credited here; in the improved
survival of AMI patients treated with Mg infusions; however its
anti-thrombotic and anti-vasoconstrictive effects may also
participate in the favorable outcome [5,8,35].
Ischemic Heart Disease
It has been recognized for many years that, with the
menopause, the death rate from ischemic heart disease (IHD)
increases, gradually rising by the seventh decade to about that
of men (Table 1 [139].
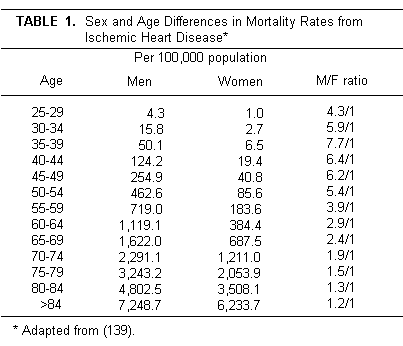
However, low dosage estrogen replacement therapy has decreased
the incidence of IHD in elderly women [140-143]. In a 1978
report, female to male ratios for pulmonary embolism death rates
were higher from 20 to about 40 years of age; thereafter they
were approximately the same [144]. A good question is whether the
higher female:male ratio of this coagulative disorder reflects
the use of OCA. OCA contained higher doses of estrogen 20 years
ago. Thrombophlebitic events in women on high dosage
estrogen-containing OCA [14-16], and in women given estrogen to
inhibit lactation [16,17], led to studies examining whether
estrogen increased coagulation. Synthetic estrogens, especially
in high doses, but not low dose conjugated estrogens were shown
to enhance blood coagulability [145-148]. In this regard, OCAs
have been found to lower serum Mg levels [149-151] and Mg
prophylaxis has been deemed protective for women on these agents
[152].
Regarding eclampsia, intravascular coagulation that is seen
with high levels of estrogen is described above. Men treated with
estrogens for prostatic carcinoma had more thrombosis-associated
cardiac disease than those not so treated [13] although the
degree of atherosclerosis seems not to have been affected [153].
OCA users who smoke have increased platelet aggregation and
decreased prostacyclin levels [154]. The interrelationships of Mg
with PGI provide an additional mechanism through which estrogen
may affect blood coagulation. It has been advised that during
long-term estrogen therapy, Mg status should be monitored and
deficits corrected to reduce the risk of phlebothrombosis
[9,155].
Marginal Clinical Magnesium
Deficiency
Women are more subject than men to latent tetany of marginal
Mg deficiency. Many developed thromboses and emboli with this
condition, yet new events have been prevented by Mg
supplementation. However, these conditions often recurred when
supplements were discontinued [152,156-158].
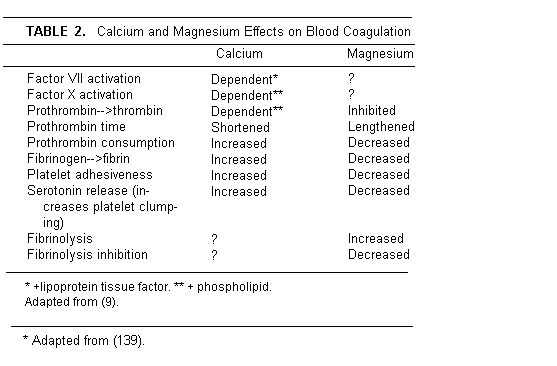
Magnesium/Calcium in Blood Coagulation
That Mg antagonizes activation Ca-induced blood clotting has
been known since 1944 [159]. By many steps, Ca leads to fibrin
formation and polymerization in clot formation has been defined
[160,161]. Interestingly, Mg counteracts Ca stimulation of
coagulation and enhances fibrinolysis [162,163] (Table
2).
Platelet aggregation requires both Mg and Ca, but is largely
Ca-dependent. Mg is needed for deaggregation and to maintenance
of platelet shape [9,164-167]. Aggregating platelets release
serotonin, which participates in vasoconstriction (supra vide).
The release is dependent on Ca and inhibited by Mg [93,168].
Serotonin-induced vascular muscle contraction is also inhibited
by Mg [92,169,170].
Partial thromboplastin and thrombin clotting times were
significantly shortened in Mg deficient calves, but prothrombin
time, platelet counts and aggregation were unaffected [171].
Increasing serum Mg only to 2.1 mEq before partial occlusion (by
suture) of coronary arteries of animals markedly diminished
platelet aggregation at the site of injury and distal to it. This
finding suggests that Mg might protect against thrombosis
formation on atherosclerotic plaques and against formation of
microthrombi associated with arteriospasms [172].
Magnesium and Experimental Lipidemic, Atherogenic, and
Thrombogenic Diets
High blood levels of Ca increase risk of arrhythmias, and
enhance blood coagulability (supra vide). Increased Mg levels
counter both. Animal experiments demonstrating the
anticoagulative, cardiovascular and renal protective effects of
adding Mg to diets designed to be thrombogenic, hyperlipidemic,
or infarctoid [3-5,173-175]. High-fat diets also have also been
shown to increase Mg requirements. The atherogenicity of high fat
diets is increased when the diets are also low in Mg [175-179].
How mechanisms involved in Mg/lipid interactions affect
coagulation and vascular disease are being elucidated [180-186].
Of significance, in relating the effects of Mg on blood lipids to
interrelationships with the effect of estrogen, is that both
estrogen and Mg function to maintain levels of high-density
lipoproteins (HDL).
Magnesium and Clinical Thrombotic Diseases
Ischemic Heart Disease. Increased platelet
adhesiveness has long been recognized in patients with IHD [187]
or AMI [188]. Adhesiveness is known to be intensified by
hyperlipemia caused by high intake of saturated fatty acids
[189-191]. As long recognized as the adverse effects of saturated
fats on blood coagulation in patients with IHD, was the favorable
response to Mg treatment of several hundred patients with IHD
with and without recent AMI [192,193]. Their reduction in angina
persisted as long as the Mg treatment was maintained, and tended
to recur when it was discontinued. A 1959 report on patients with
IHD correlated the efficacy of Mg with changes in blood lipids
and coagulation factors and thrombolysis [194]. A recent
double-blind study [195] of 47 IHD and MI patients compared the
effects of 3 months of treatment with oral Mg (15 mmol/d) with
placebo. Those receiving Mg had a 13% increase in molar ratio of
apolipoprotein A1 to apolipoprotein B, vs a 2% increase in the
placebo group. Triglycerides and very low density lipoproteins
(VLDL) decreased by 27% after Mg treatment (from 2.41 to 1.76
mmol/L, and from 1.1 to 0.79 mmol/L, respectively). Decrements
were much smaller with placebo. There was also a trend toward
increased HDL and HDL/LDL/VLDLC ratio after Mg.
Magnesium, Prostacycline and Thromboxane. It has been
hypothesized that the accelerated platelet-clumping produced by
platelet-active collagen from damaged vascular intima in Mg
deficient lambs is mediated by diminished production of PGI
[196]. The release of more arachidonic acid, the precursor of
prostanoids, from thymocytes of Mg deficient rats [197], led to
study of the effect of Mg deficiency on these derivatives of
phospholipid metabolism [198]. PGI, as measured by its stable
metabolite 6-keto-PGFa1, increased 2-fold; PGE2 increased 3-fold,
but TXB2 increased more than 10-fold. These increases were
attributed to increased Ca levels of Mg deficient rats, since the
enzyme responsible for liberation of arachidonic acid is
Ca-activated phospholipase A2 [199]. The depression of cyclic
adenosine monophosphate (cAMP) seen in Mg deficiency, also may
participate directly in the markedly increased TXB2 synthesis of
Mg deficiency, since cAMP inhibits TXB2 production by platelets
[200]. Mg deficiency alters fatty acid metabolism, with
arachidonic acid production diminished as a result of slowed
conversion of linoleic acid to arachidonic acid [201]. This
finding is pertinent to the role of prostanoids in blood
coagulation. Dietary Mg depletion of humans has been shown to
increase platelet aggregation and TXB2 release; effects that were
reversed by Mg infusion [202].
Magnesium Deficiency
and Decreased Bone Magnesium
Since osteoporosis is associated with disorders in which Mg
loss occurs, estrogen deficiency may cause loss of tissue Mg.
Postmenopausal osteoporosis is the most common form of this
bone-wasting disease. Estrogen also interacts with other hormones
that influence mineral utilization (infra vide) - both directly
and possibly through its effects on Mg [9].
Evidence suggests a low Mg state in post menopausal women with
osteoporosis. Significant retention of Mg after a parenteral Mg
load - a practical means to detect Mg deficiency [5,9,73-75] has
been found in postmenopausal patients with osteoporosis
[203,204]. Serum Mg of 10 elderly osteoporotic women was
marginally low (1.64 mEq/L) vs osteoporosis-free age-matched
women (1.74 mEq/L). Biopsied bone Mg of the osteoporotic women
(1.54 +- 0.29 mg/g) was lower than necropsy specimens from sudden
death victims (1.75 +- 0.25) (205). Mg malabsorption was
diagnosed in 12 of 20 post-menopausal osteoporotics [206]. Loss
of trabecular bone (in which the bone crystals are abnormal
[203,206]), is associated with decreased trabecular bone Mg in
postmenopausal and senile osteoporosis [203,204,206,207], in
alcohol-associated osteoporosis [208], and in diabetic
osteoporosis (208,209]. Interestingly, the same abnormalities
were not seen in estrogen treated women [203]. Patients with
non-alcoholic cirrhosis (without osteoporosis), had low Mg in
bone cortex rather than in trabecular bone [210]. The acidity of
bone extracellular fluid, postulated to fall when Mg deficiency
depresses the Mg-dependent adenosine triphosphatase H+-K+ pump in
osteocytes, may result in decline in octacalcium phosphate
formation in bone. The latter may explain the mechanism through
which long-term Mg deficiency causes osteoporosis [[211].
Conditions that Increase Magnesium Needs in
Osteoporosis
Magnesium Malabsorption and Renal Wasting. Patients
with malabsorption caused by sprue, steatorrhea, inflammatory
bowel disease, intestinal resections or genetic isolated Mg
malabsorption manifest low bone Mg and low bone Mg/Ca ratios, as
well as negative Mg balance [212-220]. Malabsorption was
associated with slight generalized bone thinning in a 67 year old
man [215], and with evidence of demineralization in a 56 year old
black woman [219]. Vertebral osteoporosis was seen in a young man
with Mg deficiency secondary to intestinal resection [213].
Juvenile osteoporosis has been reported in Bartter's syndrome, a
genetic renal Mg wasting condition [220]. Malabsorption of Mg
[207] and renal Mg wasting [221] have been diagnosed in
postmenopausal osteoporosis.
Alcoholism. Many reasons exist for alcoholics to be
hypomagnesemic. The chronic alcoholic has poor food intake and
malabsorption. Alcohol causes a diuresis which increases urinary
Mg output (seen even when consumed in moderate amounts
[222-224]), and which can cause severe enough Mg deficiency
leading to tremors, hallucinations, convulsions and arrhythmia,
all of which respond to Mg therapy [224-226]. Accordingly,
chronic Mg deficiency of alcoholism may contribute to development
of osteoporosis [208].
Pregnancy. Pregnancy increases the need for both Mg
and Ca [2,5,9,227, supra vide]. Therefore osteoporosis can be a
risk among women who have experienced multiple pregnancies. In
such women, repeated drains on Mg, as well as on Ca, may
contribute to hyperparathyroidism of pregnancy (infra vide) This
state is so common as to be termed "physiologic" [5,9,228].
Diabetes Mellitus. Mg depletion has long been
associated with decompensated diabetes mellitus (DM) [229].
Osteoporosis is a frequent complication of insulin-dependent DM
[203,230]. Subnormal serum Mg levels have been shown in juvenile
diabetics [231,232]. Contributory to Mg deficiency is glycosuria,
which increases Mg output in the urine even in normal subjects
given glucose loads [224,233]. Because insulin is important in
cellular uptake of Mg [234,235], chronic deficiency of this
hormone may diminish tissue Mg, including bone. In fact, low bone
Mg has been detected in diabetics [203,209], and low free ionic
Mg has been detected in erythrocytes of DM patients [236].
Old Age. The elderly, who are prone to involutional
osteoporosis, often have reduced food intake [237,238], impaired
intestinal Mg absorption [207,239] and increased urinary Mg
excretion [240], all factors that increase the likelihood of Mg
deficiency. Also their hormonal changes can contribute to Mg loss
[5,9,238]. Previously a dietary Ca/Mg ratio of 2/1, but with the
frequently advised intake of 1200 mg/day of Ca or more, for the
elderly, the ratio may be at least 4/1.
Calcium/Magnesium Imbalances
Effect of Increased Calcium Intakes on Magnesium. To
compensate for the loss of Ca from osteoporotic bones, oral
treatment with Ca is common. Rarely considered is the effect of
high Ca intakes on Mg requirements [5-7], or the importance of Mg
in maintaining normal bone matrix [5,9,12]. High dietary Ca/Mg
ratios interfere with Mg absorption (Figure 1),
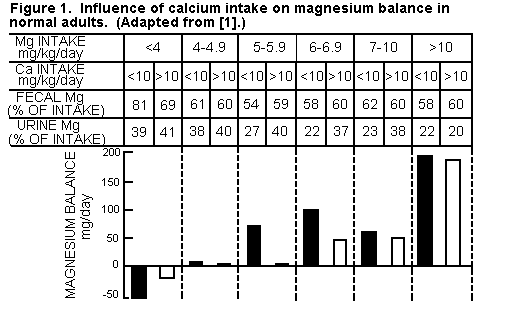
partially because Ca and Mg share a common intestinal
absorption pathway [217,241,242]. Metabolic studies have shown
interference by high dietary Ca with Mg retention of normal young
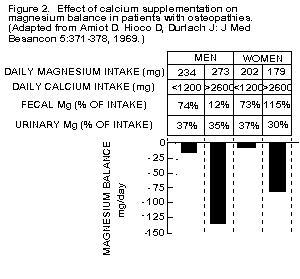
women [1,82], and of patients with osteopathies (Figure 2)
[217,243-245].
Moderately high Ca intake (1270 to 2360 mg/day) had little
effect on the Mg balance of elderly men [246]. Similarly,
increasing the Ca intake of young men from 700 to 1600 mg of Ca
had little effect on Mg balance [247]. However, plasma Mg levels
fell in the young men even though Mg balance was not affected
when they were given up to 2 g/day of Ca [248].
Early experimental studies of Mg deficiency in rats employed
diets with high Ca/Mg dietary ratios that were rich in vitamin D,
resulting in hypermineralized, brittle bones [249-251]. This may
be pertinent to the current encouragement of women to consume
substantial amounts of Ca; regard is not given to probable low
dietary Mg, as indicated by dietary surveys [252-258] and the
inadequate current RDA for Mg [81].
Effect of Magnesium Deficiency and Calcium
Supplementation. Diets low in Mg, or hypomagnesemia from
other causes [259-263] have resulted in Mg-responsive
hypocalcemia. Increasing Mg intake increased Ca absorption in
normal young men on adequate Ca intake [264]. Refractoriness of
hypocalcemia associated with Mg deficiency to Ca replacement, can
be caused by impaired release of parathyroid hormone (PTH) and by
resistance of target organs (bone and kidneys) to PTH [265-270],
and/or by failure of to form hormonally active metabolites of
vitamin D [271,272]. Treatment with Mg has long been known to
restore responsiveness to vitamin D and PTH, and to correct
hypocalcemia and hypomagnesemia [214,216,243].
Magnesium/Estrogen Interactions with Parathyroid Hormone
and Vitamin D
Loss of estrogen's antagonism of PTH activity can be inferred
by the development of postmenopausal hyperparathyroidism
[273,274], and greater severity of osteoporosis in hyper- than in
hypoparathyroid women [275]. Estrogen antagonizes PTH-induced
bone resorption [276,277], and bone of ovariectomized rats has
increased sensitivity to PTH as measured by Ca and hydroxyproline
content and decreased bone thickness [278]. Loss of estrogen,
which adversely affects Mg utilization [supra vide], is
associated with greater bone responsiveness to the Ca-mobilizing
effect of PTH, and with decreased formation of calcitriol. Both
effects enhance Ca loss [279,280]. Thus, estrogen may protect
bone via its influence on PTH release and its demineralization
effects [5,9]. Whether estrogen's influence on the parathyroids
might be mediated by Mg is not clear. As pointed out above,
response to the PTH is impaired with severe Mg deficiency, and
repair deficiency restores normal function. Paradoxically,
deficiencies of Mg insufficient to suppress release of PTH or
response of the target organs, have resulted in parathyroid
hyperplasia and bone damage typical of hyperparathyroidism in
normocalcemic calves [281] and increased PTH secretion by goat
and sheep parathyroids perfused with hypomagnesemic,
normocalcemic solutions [282-284].
Low calcitriol levels, and low activity of enzymes involved in
its formation, are associated with old age and with involutional
osteoporosis [285-290]. Nevertheless, response of the disease to
its administration is inconsistent [289]. The need for more
parathyroid stimulation for normal vitamin D metabolism by women
with osteoporosis, and the lesser response of the elderly to
calcitriol [290], has been correlated with possible Mg deficiency
[291]. Calcitriol concentration has been raised during
physiologically increased estrogen, during pregnancy [292], and
during use of estrogen [293]. It has been suggested that impaired
activation of 1-alpha-hydroxylase might be responsible for the
estrogen deficiency-induced decreased formation of the active
vitamin D metabolite [294]. On the grounds that low levels of the
active form of vitamin D were found in Mg deficiency [295], that
Mg administration (for 20 days) raised the level of PTH (the
trophic hormone for alpha-hydroxylation [296], and that estrogen
increases Mg retention (supra vide), estrogen-induced increase of
calcitriol formation might be mediated by increased tissue
Mg.
It is proposed that interrelationships between estrogen and Mg
influence both women's resistance and vulnerability to certain
diseases. When present in physiologic amounts, estrogen enhances
Mg utilization, favoring its uptake by soft and hard tissues.
Since there is substantial evidence that Mg is protective against
cardiovascular disease, it is plausible that a contributory
factor to the sex difference in prevalence of IHD in countries
where the Mg intake is marginal or low might be estrogen's
maintenance of adequate Mg levels in heart and arteries. On the
other hand, high estrogen levels - whether secreted as in
pregnancy, administered to prevent conception or lactation, or
used in the treatment of prostatic cancer - have caused
thrombotic complications. Ironically, hypercoagulability may also
be a consequence of estrogen's shift of Mg from one compartment
to another - in this case, lowering blood Mg in those with
suboptimal Mg intakes. This results in diminution of
counteraction by Mg of Ca-stimulating steps in the coagulation
cascade. Lowered blood Mg also functions to increase levels of
TXB and endothelin, and to decrease levels of PGI and EDRF.
Calcemic prophylactic therapy of osteoporosis further increases
the Ca/Mg ratio, creating a greater risk of thrombogenesis and
arterial spasms.
Mg inadequacy may play a role in migraine and premenstrual
tension, with and without participation of high estrogen levels.
That estrogen is needed for maintenance of normal bone Mg levels
is indicated by low bone Mg in post-menopausal osteoporotic bone.
Mg is needed for maintenance of normal bone structure, both
directly for matrix formation and indirectly for mineralization
through its requirement for normal parathyroid and vitamin D
metabolism. Estrogen also interacts with PTH and vitamin D,
possibly in part through its effect on Mg. Development of
osteoporosis in diseases associated with Mg loss is additional
evidence of the importance of Mg in postmenopausal women.
1. Seelig MS: The requirement of magnesium by the normal
adult. Am J Clin Nutr 14:342-390, 1964.
2. Seelig MS : Human requirements of magnesium. Factors that
increase needs. Proc First Intl Sympos on Mg Deficit in Human
Pathology, Ed J Durlach, Vittel, France 1:11-38, 1971. (privately
printed)
3. Seelig MS, Heggtveit HA: Magnesium interrelationships in
ischemic heart disease: A review. Am J Clin Nutr 27:59-79,
1974.
4. Seelig MS, Haddy FJ: Magnesium and the Arteries: I. Effects
of magnesium deficiency on arteries and on retention of sodium,
potassium, and calcium. In Magnesium in Health and Disease, eds M
Cantin, MS Seelig, Spectrum, NY,NY, 1980, pp 605-638. (2nd Intl
Mg Sympos, Quebec, Canada, 1976).
5. Seelig MS: Magnesium Deficiency in the Pathogenesis of
Disease. Early Roots of Cardiovascular, Skeletal, and Renal
Abnormalities. NY, NY, Plenum Medical Book Co. 1980
6. Seelig MS: Magnesium requirements in human nutrition.
Magnesium Bull 1a:26-47, 1981.
7. Seelig MS: Nutritional Status and Requirements of
Magnesium. Magnesium Bull 8:170-185, 1986.
8. Seelig MS: Cardiovascular consequences of magnesium
deficiency and loss: pathogenesis, prevalence and manifestations-
magnesium and chloride Loss in refractory potassium repletion. Am
J Cardiol 63:4G-21G, 1989
9. Seelig MS: Increased need for magnesium with the use of
combined oestrogen and calcium for osteoporosis treatment.
Magnesium Res:3:197-215, 1990.
10. Goldsmith NF: Physiologic relationship between magnesium
and the female reproductive apparatus. Serum magnesium variations
in normal subjects; the effect of estrogen on magnesium
distribution. Proc First Intl Sympos Mg, Ed J Durlach, Vittel,
France, 1:439-458, 1971.
11. Goldsmith NF, Johnston JO: Magnesium-estrogen hypothesis:
thromboembolic and mineralization ratios. in "Magnesium in Health
and Disease", Eds M Cantin, MS Seelig, pp 313-323, 1980.
Spectrum, New York. (Proc 2nd Intl Mg Sympos, Quebec, Canada,
1976).
12. Miller ER, Ullrey DE, Zutaut CL, Baltzer BV, Schmidt DA,
Hoefer JA, Lurcke RW: Magnesium requirements of the baby pig. J
Nutr 85:13-20, 1965.
13. Blackard CE, Doe RP, Mellinger GT, Byar DP: Incidence of
cardiovascular disease and death in patients receiving
diethylstilbestrol for carcinoma of the prostate. Cancer
26:249-256, 1970.
14. Cole M: Strokes in young women using oral contraceptives.
Arch Intern Med 120:551-555, 1967.
15. Inman WHW, Vessey MP, Westerholm B, Engelund A:
Thromboembolic disease and the steroidal content of oral
contraceptives. A report to the committee on safety of drugs.
Brit Med J 2:203-209. 1970.
16. Jeffcoate TNA, Miller J, Roos RF, Tindall VR: Puerperal
thromboembolism in relation to the inhibition of lactation by
oestrogen therapy. Brit Med J 4:19-25, 1968.
17. Unsigned Editorial: Thrombosis and inhibition of
lactation. Brit Med J 4: 1-2, 1968.
18. Hall DG: Serum magnesium in pregnancy. Obstet Gynec
9:158-162, 1957.
19. Flowers Jr CE: Magnesium sulfate in obstetrics. Am J
Obstet Gynec 91:763- 772, 775-776, 1965.
20. McGanity WJ (discussion). Magnesium sulfate in Obstetrics
Am J Obstet Gynec 91:774-775, 1965.
21. Kontopoulos V, Seelig MS, Dolan J, Berger AR, Ross RS:
Influence of parenteral administration of magnesium sulfate to
normal pregnant and preeclamptic women. In Magnesium in Health
and Disease, eds M Cantin, MS Seelig, Spectrum, NY, NY, 1980, pp
839-848. (2nd Intl Mg Sympos, Quebec, Canada, 1976).
22. Weaver K: A possible anticoagulant effect of magnesium in
preeclampsia. In In Magnesium in Health and Disease,eds M Cantin,
MS Seelig, Spectrum, NY, NY, 1980, pp 833--838. (2nd Intl Mg
Sympos, Quebec, Canada, 1976).
23. Kuti V, Balazs M, Morvay F, Varenka Z, Szekly A, Szucs M:
Effect of Maternal Magnesium Supply on Spontaneous Abortion and
Premature Birth and on Intrauterine Foetal Development:
Experimental Epidemiological Study. Magnesium Bull 3:73-79,
1981.
24. Altura BM, Altura BT, Carella A: Magnesium
deficiency-induced spasms of umbilical vessels: relation to
preeclampsia, hypertension, growth retardation. Science
221:376-378, 1983.
25. Fehlinger R, Kemnitz C, Dreibig P, Egert M, Seidel K:
[Prematurity, latent tetany and magnesium deficiency: a
retrospective study with 132 mothers.] Magnesium Bull 6:52-59,
1984. (in German)
26. Bartl W, Riss P: [Magnesium serum levels and magnesium
deficiency in pregnancy.] Magnesium Bull 6:60-62, 1984. (in
German)
27. Weaver K: Pregnancy-Induced Hypertension and Low Birth
Weight in Magnesium-Deficient Ewes. Magnesium 5: 191-200,
1986.
28. Valenzuela GJ, Munson LA: Magnesium and Pregnancy.
Magnesium 6:128-135, 1987.
29. Spaetling L, Spaetling G: Magnesium supplementation in
pregnancy; a double-blind study; after treatment with intravenous
MgSO4: a preliminary report. Br J Obstet Gynec 95:120-125,
1988.
30. De Ridder G, Gossen D: Magnesium in
pregnancy--relationship to prophylaxis and therapy of early
contractions and threatening premature birth. Magnesium Res.
1:247, 1988.
31. Kovacs L, Molnar BG, Huhn E, Bodis L: [Magnesium
substitution in pregnancy. A prospective, randomized double-blind
study]. Geburtshilfe-Frauenheilkd. 48:595-600, 1988. (in
German)
32. Sjogren A, Gennser G, Rymark P: Reduced concentrations of
magnesium, potassium and zinc in skeletal muscle from women
during normal pregnancy or eclampsia. J Amer Coll Nutr 7:408,
1988.
33. LeBouedec G, Begon E, Monteillard C, Gioanni G, Pignide L,
Bruhat MA: [Magnesium and the threat of premature labor]. J
Gynecol Obstet Biol Reprod Paris 18: 53-60, 1989. (in French)
34. Molnar BG, Kovacs L: Experiences with magnesium
substitution during pregnancy. Magnesium Res 2:230-231, 1989.
35. Woods K.L.: Possible pharmacological actions of magnesium
in acute myocardial infarction. Br J Clin Pharmacol 32:3-10,
1991.
36. Lazard EM: A preliminary report on the intravenous use of
magnesium sulphate in puerperal eclampsia. Am J Obstet Gynec,
9:178-188, 1925.
37. Sjollema B: Nutritional and metabolic disorders in cattle.
Nutr Abstr Rev 1:621-632, 1932.
39. Sibai BM: Magnesium sulfate is the ideal anticonvulsant in
preeclampsia- eclampsia. Am J Obstet Gynecol 162:1141-1145,
1990.
40. Elliott JP: Magnesium sulfate as a tocolytic agent. Am J
Obstet Gynecol 147:277-284. 1983.
41. Valenzuela G, Hayashi RH, Johns A: Effect of magnesium
sulfate upon uterine contractility in humans. Magnesium
2:120-124, 1983.
42. Classen HG, Helbig J: [Magnesium in obstetrics and
gynecology.] Magnesium Bull 6:45-51, 1984.
43. Spaetling L, Schneider H, Huch R, Huch A: [Magnesium as an
additional therapy to tocolysis.] Magnesium Bull 6:63-67,
1984.
44. Conradt A, Weidinger H, Algayer H: Magnesium therapy
decreased the rate of intrauterine fetal retardation, premature
rupture of membranes and premature delivery in risk pregnancies
treated with betamimetics. Magnesium 4:20-28, 1985.
45. Wischnik A, Weidinger H: [Magnesium Aspartate and
Magnesium Sulfate in Obstetrics.] Magnesium Bull 7:96-101, 1985.
(In German)
46. Spaetling L: Magnesium deficiency and premature labour.
Magnesium Bull 7: 81-86, 1985.
47. Spaetling L: Magnesium deficiency and preterm labor. In
Magnesium in Health and Disease, eds Y Itokawa, J Durlach. J.
Libbey, London, 1989, pp 363-366 (5th Intl Mg Sympos, Kyoto,
1988).
48. Hatjis CG, Swain M, Nelson LH, Meis PJ, Ernest JM:
Efficacy of combined administration of magnesium sulfate and
ritodrine in the treatment of premature labor. Obstet Gynecol
69(3 Pt 1): 317-322, 1987.
49. Hollander DI, Nagey DA, Pupkin MJ: Magnesium sulfate and
ritodrine hydrochloride: a randomized comparison. Am J Obstet
Gynec 156:631-637, 1987.
50. Bonnar J, McNicol GP, Douglas AS: Coagulation and
fibrinolytic systems in preeclampsia and eclampsia. Brit J Med
3:12-16, 1971.
51. Unsigned Editorial: Antenatal thromboembolism. Brit Med J
1:249-250, 1970.
52. Warkany J, Monroe BB, Sutherland BS: Intrauterine growth
retardation, J Dis Child 102:249-279, 1961.
53. McKay DG, Goldenberg V, Kaunitz H, Csvassy I: Experimental
eclampsia. An electron microscopic study and review. Arch Path
84:557-597, 1967.
54. Miller JK, Schneider MD, Ramsey N, White PK, Bell MC:
Effects of hypomagnesemia on reactivity of bovine and ovine
platelets: possible relevance to infantile apnea and sudden
infant death syndrome. J Am Coll Nutr 9: 58-64, 1990.
55. Weaver K: Magnesium and its role in vascular reactivity
and coagulation. Contemp Nutr 12:1-2, 1987.
56. Weaver K: Magnesium and migraine: reversible
hypomagnesemic coagulative angiopathy. Hypothesis and preliminary
clinical data. J Am Coll Nutr 1:187-188, 1983.
57. Ogburn PL, Williams PP, Johnson SB, Holman RT: Serum
arachidonic levels in normal and preeclamptic pregnancies. Am J
Obstet Gynecol 148:5-9, 1984.
58. Watson KV, Moldow CF, Ogburn PL, Jacob HS: Magnesium
sulfate: rationale for its use in preeclampsia. Proc Natl Acad
Sci USA 83:1075-1078, 1986.
59. Briel RC, Lippert TH, Zahradnik HP: [Changes in blood
coagulation, thrombocyte function and vascular prostacyclin
synthesis caused by magnesium sulfate]. Geburtshilfe Frauenheilkd
47:332-336, 1987. (in German)
60. Mastrogiannis DS, O'Brien WF, Krammer J, Benoit R:
Potential role of endothelin-1 in normal and hypertensive
pregnancies. Am J Obstet Gynecol 165(6 Pt 1):1711-1716, 1991.
61. Wynn A, Wynn M: Magnesium and other nutrient deficiencies
as possible causes of hypertension and low birthweight. Nutr
Health. 6:69-88, 1988.
62. Losonczy J, Adorjan G, Novak M, Toth MO: Correlation
between the incidence of preterm delivery and the concentration
of magnesium in drinking water in Szabolcs-Szatmar County,
Northeast Hungary. Magnesium Res 2:229-230, 1989.
63. Novak M, Losonczy J, Kornafeld J, Toth MO: Experiences on
giving magnesium citrate to pregnant mothers. Magnesium Res
2:230, 1989.
64. Borella P, Szilagyi A, Than G, Csaba I, Giardino A,
Facchinetti F: Maternal plasma concentrations of magnesium,
calcium, zinc and copper in normal and pathological pregnancies.
Sci Total Environ 99:67-76, 1990.
65. Sibai BM, Villar MA, Bray E: Magnesium supplementation
during pregnancy: a double-blind randomized controlled clinical
trial. Am J Obstet Gynecol 161:115-119, 1989.
66. Coons C, Blunt K: retention of nitrogen, calcium,
phosphorus, and magnesium by pregnant women. J Biol Chem 86:1-16,
1930.
67. Coons CM, Schiefelbusch AT, Marshall BB, Coons RR: Studies
in metabolism during pregnancy. Oklahoma Agric Mech Coll Agric
Exp Sta Bull #223: 1-113, 1935.
68. Hummel F, Sternberger H, Hunscher H, Macy I: Metabolism of
women during the reproductive cycle. VII. Utilization of
inorganic elements (a continuous case study of a multipara). J
Nutr 11:235-255, 1936.
69. Hummel FC, Hunscher HA, Bates MF, Bonner P, Macy IC,
Johnston JA: A consideration of the nutritive state in the
metabolism of women during pregnancy. J Nutr 13:263-278, 1937
70. Duggin GG, Dale NE, Lyneham RC, Evans RA, Tiller DJ:
Calcium balance in pregnancy. Lancet (Oct): 926-927, 1974.
71. Ashe JR, Schofield FA, Gram MR: The retention of calcium,
iron, phosphorus, and magnesium during pregnancy: the adequacy of
prenatal diets with and without supplementation. Am J Clin Nutr
32:286-291, 1979.
72. Franz KB: Correlation of Urinary Magnesium Excretion with
Blood Pressure of Pregnancy. Magnesium Bull 4:73-78, 1982.
73. Elin R.J. (1988): Magnesium metabolism in health and
disease. Disease-a- Month 34:161-219
74. Palla GP, Giaquinto P, Moro PR, Maniccia E, Carelli G,
Mancuso S: Magnesium Load Test in Pregnancy Hypertension. Clin
Exp Hypertens Pregn B7:159-163, 1988.
75. Palla GP, Castaldo F, Moro P R, Giaquinto P, Carelli G,
Caruso A, Lanzone A, Mancuso S: Intravenous magnesium load test
in normal and diabetic pregnant women. Magnesium Res 2:91-92,
1989.
76. Johnson NE, Philipps C: Magnesium content of diets of
pregnant women. In Magnesium in Health and Disease. eds M Cantin,
MS Seelig, Spectrum, New York, 1980, pp 827-831. (2nd Intl Sympos
on Magnesium, Quebec, 1976) 77. Endres J, Dunning S, Poon S,
Welch P, Duncan H: Older pregnant women and adolescents:
Nutrition data after enrollment in WIC. J Amer Dietetic Assc
87:1011-1019, 1987.
78. Franz KB: Magnesium intake during Pregnancy Magnesium
6:18-27, 1987.
79. McGarvey ST, Zinner SH, Willett WC, Rosner B: Maternal
prenatal dietary potassium, calcium, magnesium, and infant blood
pressure. Hypertension 17:218-224, 1991.
80. Food and Nutr Bd: Recommended Dietary Allowances, 9th Ed,
Natl Acad Press, pp 134-136, 1980.
81. Food and Nutr Bd: Recommended Dietary Allowances, 10th Ed,
Natl Acad Press, pp 187-194, 1989.
82. Hathaway M: Magnesium in human nutrition. Home Econ Res
Rep 19, Agric Res Serv, USDA, 1962.
83. Irwin MI, Feeley RM: Frequency and size of meals and serum
lipids, nitrogen and mineral retention, fat digestibility and
urinary thiamine and riboflavin in young women. Am J Clin Nutr
20:816-824, 1967.
84. Seelig MS: Magnesium in pregnancy: special needs for the
adolescent mother. J Am Coll Nutr 10:566, 1991.
85. Seelig MS, Franz K, Weaver K: Poor outcomes of adolescent
pregnancy; relationship to magnesium deficiency. (in
preparation)
86. Rotton WN, Sachtleben MR, Friedman EA: Migraine and
eclampsia. Obstet Gynecol 14:322-330, 1959.
87. Weaver K (letter to editor): Migraine and magnesium.
Perspect Biol Med 33:150-151, 1989.
88. Moore MP, Redman CWG: Case control study of severe
eclampsia of early onset. Brit Med J 287:580-583, 1983.
89. Swanson DR: Migraine and magnesium; eleven neglected
connections. Perspect Biol Med 31:526-557, 1988.
90. Horrobin DF: Hypothesis: prostaglandins and migraine.
Headache 17:113-117, 1977.
91. Anthony M: Serotonin and cyclic nucleotides in migraine.
Adv Neurol 33:45-49, 1982.
92. Allen GS, Gross CJ, Henderson LM, Chou SN: Cerebral
arterial spasm. IV. In vitro effects of temperature, serotonin
analogues, large nonphysiologic concentrations of serotonin, and
extracellular calcium and magnesium on serotonin-induced
contractions of the canine basilar artery. J Neurosurg
44:585-593, 1976.
93. Sneddon JM, Willimas KI: Effect of cations on the platelet
release reaction. J Physiol (Lond) 235:625-637, 1973.
94. Itokawa Y, Tanaka C, Kimura M: Effect of thiamine on
serotonin levels in magnesium-deficient animals. Metabolism
21:375-379, 1972.
95. Olesen J: Role of calcium-entry blockers in the
prophylaxis of migraine. Europ Neurol 25 (suppl 1):72-79,
1986.
96. Iseri LT, French JH: Magnesium: nature's physiologic
calcium blocker. Am Heart J 108:188-193, 1984.
97. Altura BM (editorial): Calcium antagonist properties of
magnesium; implication for antimigraine actions. Magnesium
4:269-275, 1985.
98. Abraham GE, Lubran MM: Serum and red cell magnesium levels
in patients with premenstrual tension. Am J Clin Nutr
34:2364-2366, 1981.
99. Abraham GE: Magnesium deficiency in premenstrual tension.
Magnesium Bulletin 4:68-73, 1982.
100. Facchinetti F, Borella P, Valentini M, Pironti T, Ficroni
L, Genazzani A: Magnesium variability in blood cells throughout
the normal and pathological menstrual cycle. in Magnesium in
Health and Disease eds Y Itokawa, J Durlach, Publ: J Libbey,
London, 1989, pp. 163-170 (Fifth Intl Mg Sympos, Kyoto, Japan
1988)
101. Stewart A, Howard J: Magnesium and potassium deficiencies
in women with pre-menstrual syndrome. Magnesium Bull 8:314-316,
1986.
102. Abraham GE: Nutritional factors in the etiology of the
premenstrual syndrome tension syndromes. J Reprod Med 28:446-464,
1983.
103. Abraham GE: Nutrition and the premenstrual tension
syndromes. J Appl Nutr 36:103-124, 1984.
104. Gallant MP, Bowering J, Short SH, Turkki PR: Pyridoxine
and Magnesium Status of Women with Premenstrual Syndrome.
Nutrition Research 7:243-252, 1987.
105. Facchinetti F, Borella P, Sances G, Fioroni L, Nappi RE,
Genazzani AR Oral magnesium successfully relieves premenstrual
mood changes. Obstet Gynecol 78:177-181, 1991.
106. Abraham GE, Rumley RE: Role of nutrition in managing the
premenstrual tension syndromes. J Reprod Med 32:405-422,
1987.
107. London RS, Bradley L, Chiamori NY: Effect of a
nutritional supplement on premenstrual symptomatology in women
with premenstrual syndrome: a double-blind longitudinal study. J
Am Coll Med 10:494-499, 1991.
108. Kruse HD, Orent ER, McCollum V: Studies on magnesium
deficiency in animals. Symptomatology resulting from magnesium
deprivation. J Biol Chem 96:519-539, 1932.
109. Greenberg D, Tufts E: The nature of magnesium tetany. Am
J Physiol 121:269-300, 1938.
110. Seta K, Hellerstein E, Vitale JJ: Myocardium and plasma
electrolytes in dietary magnesium and potassium deficiency in the
rat. J Nutr 87:179-188, 1965.
111. Seta K, Kleiger R, Hellerstein EE, Lown B, Vitale JJ:
Effect of potassium and magnesium deficiency on the
electrocardiogram and plasma electrolytes of pure-bred beagles.
Am J Cardiol 17:516-519, 1966.
112. Syllm-Rapaport I, Strassburger I, Gruneberg D, Zirbel C:
Electrocardiographic studies in dogs with experimental magnesium
deficiency. J Paediat 60:801-804, 1962.
113. Wener J, Pintar K, Simon MA, Motola R, Friedman R, Mayman
A, Schucher R: The effects of prolonged hypomagnesemia on the
cardiovascular system in young dogs. Am Heart J 67:221-231,
1964.
114. Vitale JJ, Hellerstein EE, Nakamura M, Lown B: Effects of
magnesium deficient diet upon puppies. Circ Res 9:387-394,
1961.
115. Vitale JJ, Velez H, Guzman C, Correa P: Magnesium
deficiency in the cebus monkey. Circ Res 12:642-650, 1963.
116. Berliner K: Then effect of calcium injections on the
human heart. Am J Med Sci 191:117-121, 1936.
117. Bernstein M, Simkins S: Magnesium; effects of intravenous
injections on human heart. J Lab Clin Med 25:131-141, 1939.
118. Zwillinger L: [About magnesium's effect on the heart].
Klin Wschr 14:1429-1433, 1935. (in German)
119. Iseri LT, Freed J, Bures AB: Magnesium deficiency and
cardiac disorders. Am J Med 58:837-846, 1975.
120. Dyckner T, Wester PO: Ventricular extrasystoles and
intracellular electrolytes before and after potassium and
magnesium infusions in patients on diuretic treatment. Am Heart J
97:12-18, 1979.
121. Dyckner T, Wester PO: Potassium/Magnesium Depletion in
Patients with Cardiovascular Disease. Am J Med 82(3A):11-17,
1987.
122. Charbon GA: Magnesium treatment of arrhythmia: drug or
nutritional replenishment? pitfalls for the experimental design.
In Magnesium in Health and Disease, eds Y Itokawa J Durlach, Publ
J Libbey, London, 1989, pp 223-228. (Fifth Intl Mg Sympos, Kyoto,
Japan,1988).
123. Iseri LT: Role of magnesium in cardiac tachyarrhythmias.
Am J Cardiol 65: 47K-50K, 1990.
124. Morton BC, Nair RC, Smith FM, McKibbon TG, Poznanski WJ:
Magnesium in therapy in acute myocardial infarction - a
double-blind study. Magnesium 3:346-352, 1984.
125. Smith LF, Heagerty AM, Bing RF, Barnett DB: Intravenous
infusion of magnesium sulphate after acute myocardial infarction:
effects on arrhythmias and mortality. Intl J Cardiol 12:175-183,
1986.
126. Rasmussen HS: Justification for intravenous magnesium
therapy in acute myocardial infarction. Magnesium Res 1:59-73,
1988.
127. Rasmussen HS, Gronbaek M, Cintin C, Balslov S, Norregard
P, McNair P: One-year death rate in 270 patients with suspected
acute myocardial infarction, initially treated with intravenous
magnesium or placebo. Clin Cardiol 11: 377-381, 1988.
128. Golf SW, Temme H, Moebius T, Greaf V, Roka L, Homann J:
magnesium therapy after acute myocardial infarction: chronologic
sequence of biochemical blood parameters. Magnesium Bull
10:"119-123, 1988.
129. Bertschat F, Ising H, Guenther T, Sorgenfrei J, Wollitz
M, Ibe K: Antiarrhythmic effects of magnesium infusions in
patients with acute myocardial infarction. Magnesium Bull
11:155-158, 1989.
130. Rasmussen HS: Clinical intervention studies on magnesium
in myocardial infarction. Magnesium 8:316-325, 1989.
131. Sheehan J: Importance of magnesium chloride repletion
after myocardial infarction. Am J Cardiol 53:35G-38G, 1989.
132. Abraham AS: Treatment of patients with acute myocardial
infarction with intravenous magnesium. Magnesium Trace Elem
9:177-185, 1990.
133. Pereira D, Pereira TG, RabaÝal C, Carvalho E,
Linder J, Afonso JS, Pereira JN, Halpern MJ, Fernandes JS:
[Effect of intravenous administration of SO4Mg in the acute phase
of myocardial infarct]. Rev Port Cardiol 9:205-210, 1990.
134. Shechter M: Beneficial Effect of magnesium in acute
myocardial infarction. A Review of the Literature. Magnesium Bull
12: 1-6, 1990.
135. Shechter M, Hod H, Marks N, Behar S, Kaplinsky E,
Rabinowitz B: Beneficial effect of magnesium sulfate in acute
myocardial infarction. Am J Cardiol 66:271-274, 1990.
136. Singh RB, Sircar AR, Rastogi SS, Garg V: Magnesium and
potassium administration in acute myocardial infarction.
Magnesium Trace Elem 9:198-204, 1990.
137. Teo KK, Usuf S, Collins R, Held PH, Peto R: Effects of
intravenous magnesium in suspected acute myocardial infarction:
overview of randomized trials. Brit Med J 303:1499-1503,
1991.
138. Rebentisch E, Ising H,Bertschat F, Sorgenfrei J:
(Magnesium treatment during arrhythmias following myocardial
infarction - therapeutic efficacy and electrolyte status.)
Magnesium Bull 14:111-121, 1992. (in German)
139. Stamler JS: The relationship of sex and gonadal hormones
to atherosclerosis. in Atherosclerosis and its Origin. eds M
Sandler, GH Bourne, Academic Press, NY, 1963, pp 231-262.
140. Bush TL, Barrett-Connor E: Noncontraceptive estrogen use
and cardiovascular disease. Epidemiol Rev 7:80-104, 1985.
141. Stampfer MJ, Willet WC, Coldita GA, Rosner B, Speizer FE,
Henekens CH: A prospective study of postmenopausal estrogen
therapy and coronary heart disease. New Engl J Med 313:1044-1049,
1985.
142. Knopp RH: Arteriosclerosis risk. The roles of oral
contraceptives and postmenopausal estrogens. J Reprod Med
31:913-921, 1986.
143. Henderson BE, Paganini-Hill A, Ross RK: Estrogen
replacement therapy and protection from acute myocardial
infarction. Am J Obstet Gynec 159: 312-317, 1988.
144. Anderson TW: The changing pattern of ischemic heart
disease. New Scientist 9:374-376, 1978.
145. Bolton CH, Hampton JR, Mitchell JRA: Effect of oral
contraceptive agents on platelets and plasma phospholipids.
Lancet 1:1336-1341, 1960.
146. Robinson RW, LeBeau R, Maschouf C: Effect of conjugated
equine estrogens on the clotting mechanism. Circulation 28:670
(abstr), (1963.
147. Caspary EA, Peberdy M: Oral Contraception and Blood
Platelet Adhesiveness. Lancet 1: 1142-1143, 1965.
148. Paganini-Hill A, Ross RK, Henderson BE: Postmenopausal
oestrogen treatment and stroke: a prospective study. Brit Med J
297:519-522, 1988.
149. Goldsmith NF, Pace N, Baumberger JP, Ury H: Magnesium and
citrate during the menstrual cycle. Effect of an oral
contraceptive on serum magnesium. Fertil Steril 21:292-300,
1970.
150. Goulding A, McChesney R: Oestrogen-progestogen oral
contraceptives and urinary calcium excretion. Clin Endocr
6:449-454, 1977.
151. Olatunbosun DA, Adeniyi FA, Adadevoh BK: Effect of oral
contraceptives on serum magnesium levels. Intl J Fert 19:224-226,
1974.
152. Durlach J: The pill and thrombosis; platelets, estrogen
and magnesium. Rev Franc Endocr Clin 11:45-54, 1970. (in
French)
153. Hanash KA, Taylor WF, Greene LF, Koeke BA, Titus JL:
Relationship of estrogen therapy for carcinoma of the prostate to
atherosclerotic cardiovascular disease: a clinicopathologic
study. J urol103:467-470, 1970.
154. Mileikowsky GN, Nadler JL, Francis R, Roy S: Evidence
that smoking alters prostacyclin formation and platelet
aggregation in women who use oral contraceptives. Am J Obstet
Gynecol 159:1547-1552, 1988.
155. Durlach J: Clinical aspects of chronic magnesium deficit.
In Magnesium in Health and Disease, eds M Cantin, MS Seelig,
Spectrum, NY, NY, 1980, pp 883--909. (2nd Intl Mg Sympos, Quebec,
Canada, 1976).
156. Durlach J: [The antithrombotic physiology of magnesium;
phlebothrombosis caused by magnesium deficiency.] Coeur Med
Interne 6:213-232, 1967. (in French)
157. Dupont B, Pony JC, LeBihan G, Leborgne P:
[Phlebothrombotic disease and magnesium.] Sem Hop Paris
45:3048-3054, 1969.
158. Maurat J-P, Debrand J, Kieffer Y, Cardot N : [Magnesium
deficiency and platelet aggregability.] (French) Rev Franc
Endocrinol Clin 15:505-508, 1974. (in French)
159. Greville GD, Lehmann H: Cation antagonism in blood
coagulation. J Physiol 103:175-184, 1944.
160. Silver MJ: Role of calcium ions and phospholipids in
platelet aggregation and plug formation. Am J Physiol
209:1128-1136, 1965.
161. Lorand L, Konishi K: Activation of the fibrin stabilizing
factor of plasma by thrombin. Arch Biochem Biophys 105:58-67,
1964.
162. Zahnert R, Oloffs J: [Study of the role of magnesium
coagulation factors and the thromboelastogram]. Dtsche-Gesundh
15:2343-2348, 1960 (in German)
163. Huntsman RG, Hurn BA, Lehmann H: Observations on the
effect of magnesium on blood coagulation. J Clin Path 13:99-101,
1960.
164. Herrmann RG, Lacefield WB, Crowe VG: Effect of ionic
calcium and magnesium on human platelet aggregation. Proc Soc Exp
Biol Med 135: 100-103, 1970.
165. Born GVR, Cross MJ: Effects of inorganic ions and of
plasma proteins on the aggregation of blood platelets by
adenosine phosphate. J Physiol 170: 397-414, 1964.
166. Elin RJ: Role of magnesium in membranes: erythrocyte and
platelet function and stability. In Magnesium in Health and
Disease,eds M Cantin, MS Seelig, Spectrum, NY, NY, 1980, pp
113-124.(2nd Intl Mg Sympos, Quebec, Canada, 1976).
167. Hughes A, Tonks RS: Magnesium, adenosine diphosphate and
blood platelets. Nature 210:106-107, 1966.
168. Penglis F, Michal F: The induction of blood platelet
aggregation by divalent cations. Experimentia 25:745-746,
1969.
169. Altura BM, Altura BT: Mg, Na, and K interactions and
coronary artery diseases. Magnesium 1:241-256, 1982.
170. Goldstein S, Zsoter TT: The effect of magnesium on
response of smooth muscle to 5-hydroxytryptamine. Br J Pharmacol
62:507-514, 1978.
171. Stevenson MM, Yoder I: Studies of platelet aggregation,
plasma adenosine diphosphate breakdown and blood coagulation in
magnesium deficient calves and rats. Thromb Diath Hemorrh
23:299-305, 1979.
172. Gertz SD, Wajnberg RS, Kurgon A, Uretzky G: Effect of
magnesium sulfate on thrombus formation following partial
arterial constriction: implications for coronary vasospasm.
Magnesium 6: 225-235, 1987.
173. Vitale J, Hellerstein E, Hegsted D, Nakamura M, Farbman
A: Studies on the interrelationships between dietary calcium and
magnesium in atherogenesis and renal lesions. Am J Clin Nutr
7:13-22, 1959.
174. Bunce G, Chiemchaisri Y, Phillips P: The mineral
requirements of the dog. IV. Effect of certain dietary and
physiologic factors upon the magnesium deficiency syndrome. J
Nutr 76:23-29, 1962.
175. Sos J, Gati T, Kemeny T, Rigo J: Infarctoid cardiac
lesions induced by dietetic factors in the dog. Acta Med Acad Sci
Hung 20:1-8, 1964.
176. Vitale J, White P, Nakamura M, Hegsted D: Effect of
feeding an atherogenic diet on magnesium requirement. Fed Proc
16:400-401, 1957.
177. Vitale J, White P, Nakamura M, Hegsted D, Zamcheck M,
Hellerstein E: Interrelationships between experimental
hypercholesterolemia, magnesium requirements and experimental
atherosclerosis. J Exp Med 106:757-766, 1957.
178. Szelenyi I, Rigo J, Ahmed BO, Sos J: The role of
magnesium in blood coagulation. Thromb Diath Haemorrh 18:626-633,
1967.
179. Savoie LL: [Role of magnesium, potassium, antikaluretics
and hypocholesterolemics in development of cardiac lesions in the
rat fed a thrombogenic diet with NaH2PO4.] Path Biol 20:19-20,
751-756, 1972.
180. Rayssiguier Y: Magnesium and lipid interrelationships in
the pathogenesis of vascular diseases. Magnesium Bull 3:165-177,
1981.
181. Gueux E, Rayssiguier Y: The hypercholesterolaemic effect
of magnesium efficiency following cholesterol feeding in the rat.
Magnesium Bull 3: 126-129, 1981.
182. Rayssiguier Y, Gueux E: The reduction of plasma
triglyceride clearance by magnesium-deficient Rats. Magnesium
2:132-138, 1983.
183. Gueux E, Rayssiguier Y, Piot M-C, Alcindor L: The
reduction of plasma lecithin-cholesterol acyl-transferase
activity by acute magnesium deficiency in the rat. J Nutr
114:1479-1483, 1984.
184. Rayssiguier Y: Magnesium, lipids and vascular diseases.
Experimental evidence in animal models. Magnesium 5:182-190,
1986.
185. Rayssiguier Y, Gueux E: Magnesium and lipids in
cardiovascular disease. J Am Coll Nutr 5:507-519, 1986.
186. Rayssiguier Y, Gueux E, Cardot P, Thomas G, Robert A,
Trugnan G: Variations of fatty acid composition in plasma lipids
and platelet aggregation in magnesium deficient rats. Nutr Res
6:233-240, 1986.
187. McDonald L, Edgill M: Dietary restriction and
coagulability of the blood in ischaemic heart disease. Lancet
1:996-998, 1958.
188. Hughes A, Tonks RS: Platelets, magnesium, myocardial
infarction. Lancet 1:1044-1046, 1965.
189. Horlick L: Platelet adhesiveness in normal persons and in
subjects with atherosclerosis. Effect of high fat meals and
anticoagulants in the adhesive Index. Am J Cardiol 8:459-470,
1961.
190. Mustard JF, Murphy EA: Effect of different dietary fats
on blood coagulation, platelet economy, and blood lipids. Brit
Med J 1: 1651-1655, 1962.
191. Connor WE: The acceleration of thrombus formation by
certain fatty acids. J Clin Invest 41:1199-1205, 1962.
192. Malkiel-Shapiro B, Bershon I, Terner PE: Parenteral
magnesium sulphate therapy in coronary heart disease. A
preliminary report on its clinical and laboratory aspects. Med
Proc 2:455-462, 1956.
193. Malkiel-Shapiro B: Further observations on parenteral
magnesium sulphate therapy in coronary heart disease: a clinical
appraisal. S African Med J 32:1211-1215, 1958.
194. Parsons RS, Butler TC, Sellars EP: The treatment of
coronary artery disease with parenteral magnesium sulphate. Med
Proc 5:487-498, 1959.
195. Rasmussen HS, Aurup P, Goldstein K, McNair P, Mortensen
P, Larsen OG, Lawaetz H: Influence of magnesium substitution
therapy on blood lipid composition in patients with ischemic
heart disease. A double-blind, placebo controlled study. Arch
Intern Med 149:1050-1053, 1989.
196. Schneider MD, Miller JK, White PK, Ramsey N: Life span
and tissue distribution of 111indium-labeled blood platelets in
hypomagnesemic lambs. Magnesium Bull 3:116-122, 1981.
197. Averdunk R, Guenther T: Phospholipid metabolism and
concavalin. A stimulation of thymocytes from magnesium deficient
rats and magnesium - deficiency induced lymphoma. Magnesium Bull
7:11-15, 1985.
198. Nigam S, Averdunk R, Guenther T: Alteration of prostanoid
metabolism in rats with magnesium deficiency. Prostaglandins
Leukotrienes Med 23:1-10, 1986.
199. Van den Busch H: Intracellular phospholipase A. Biochim
Biophys Acta 604:191-246, 1980.
200. Malmsten C, Granstom E, Samuelsson B: Cyclic-AMP inhibits
synthesis of prostaglandin endoperoxide (PGG2) in Human
Platelets. Biochem Biophys Res Commun 68:569, 1976.
201. Mahfouz MM, Kummerow FA: Effect of magnesium deficiency
on delta 6-desaturase activity and fatty acid composition of rat
liver microsomes. Tech 13: . (1990) (In press)
202. Nadler J, Kuong H, Ehrlich L, Ryzen E, Frances R, Rude R:
Magnesium plays an important role in the regulation of
thromboxane release and platelet aggregation. presented: 1989
Natl Mtg Am Fed Clin Res, Washington DC 203. Cohen L, Laor A,
Kitzes R: Bone magnesium, crystallinity index and state of body
magnesium in subjects with senile osteoporosis, maturity onset
diabetes and women treated with contraceptive preparations.
Magnesium 2: 70-75, 1983.
204. Cohen L: Recent data of magnesium and osteoporosis.
Magnesium Res 1:85- 87, 1988.
205. Reginster JY, Strause L, Deroisy R, Lecart MP, Saltman P,
Franchimont P: Preliminary report of decreased serum magnesium in
postmenopausal osteoporosis. Magnesium 8:106-109, 1989.
206. Cohen L, Kitzes R: Infrared spectroscopy and magnesium
content of bone mineral in osteoporotic women. Israel J Med Sci
17:1123-1125, 1981.
207. Cohen L, Laor A, Kitzes R: Magnesium malabsorption in
postmenopausal osteoporosis. Magnesium 2:139-143, 1983.
208. Cohen L, Laor A, Kitzes R: Lymphocyte and bone magnesium
in alcohol-associated osteoporosis. Magnesium 4:148-152,
1985.
209. De Leeuw I, Vertonnen J: The magnesium content of the
trabecular bone in diabetic subjects. Biomedicine 29:16-17,
1968.
210. Cohen L, Kitzes R: Relationship of bone and plasma
magnesium in magnesium deficient cirrhosis patients. Israel J Med
Sci 18:679-682, 1982.
211. Driessens FCM, Verbeeck RMH, vanDijk JWE, Borggreven
JMPM: Response of plasma calcium and phosphate to magnesium
depletion. A review and its physiological interpretation.
Magnesium Bull 9:193-201, 1987.
212. Hanna S, Harrison M, MacIntyre I, Fraser R: The syndrome
of magnesium deficiency in man. Lancet 2:172-175, 1960.
213. Booth CC, Babouris MB, Hanna S, Maclntyre I: Incidence of
hypomagnesemia in intestinal malabsorption. Brit Med J 2:141-143,
1963.
214. Petersen VP: Metabolic studies in clinical magnesium
deficiency. Acta Med Scand 173:285-298, 1963.
215. Fishman RA: Neurological aspects of magnesium metabolism.
Arch Neurol 12:562-569, 1965.
216. MacIntyre I, Hanna S, Booth CC, Read AE: Intracellular
magnesium deficiency in Man. Clin Sci 20:297-305, 1961.
217. Heaton FW, Fourman P: Magnesium deficiency and
hypocalcaemia in intestinal malabsorption. Lancet 2: 50-52,
1965.
218. Nordio S, Donath A, Macagno F, Gatti R: Chronic
hypomagnesemia with magnesium-dependent hypocalcemia. Acta
Paediat Scand 60:441-455, 1971.
219. Connor TB, Toskes P, Mahaffey J, Martin LG, Williams JB,
Walser M: Parathyroid function during chronic magnesium
deficiency. Johns Hopkins Med J 131:100-117, 1972.
220. Sann L, Moreau P, Longin B, Sassard J, Francois R: Un
syndrom de Bartter associant un hypercortisolisme, un diabete
phosphore et magnesium, et une tubulopathie d'origine familiale.
Arch Franc Ped 32:349-366, 1975.
221. Seelig MS, Berger AR, Avioli LA: Speculations on renal,
hormonal, and metabolic aberrations in a patient with marginal
magnesium deficiency. In Magnesium in Health and Disease,eds M
Cantin, MS Seelig, Spectrum, NY, NY, 1980, pp 459-468 (2nd Intl
Mg Sympos, Quebec, Canada, 1976).
222. Kalbfleisch JM, Lindeman RD, Ginn HE, Smith WO: Effects
of ethanol administration on urinary excretion of magnesium and
other electrolytes in alcoholic and normal subjects. J Clin
Invest 42:1471-1475, 1963.
223. Mendelson JH, Ogata M, Mello N: Effects of alcohol
ingestion and withdrawal on magnesium states of alcoholics:
clinical and experimental findings. Ann N Y Acad Sci 162:918-933,
1969.
224. Lindeman RD.: Nutritional influences on magnesium
homeostasis with emphasis on renal factors. In Magnesium in
Health and Disease,eds M Cantin, MS Seelig, Spectrum, NY,NY,
1980, pp 381-399 (2nd Intl Mg Sympos, Quebec, Canada, 1976).
225. Flink EB, Stutzman FL, Anderson AR, Konig T, Fraser R:
Magnesium deficiency after prolonged parenteral fluid
administration and after chronic alcoholism complicated by
delirium tremens. J Lab Clin Med 43:169-183, 1954.
226. Flink EB: Magnesium deficiency in alcoholism. Alcoh: Clin
Exp Res 10:590- 594, 1986.
227. Seelig MS: Prenatal and neonatal mineral deficiencies:
magnesium, zinc, and chromium. in Clinical Disorders in Pediatric
Nutrition, ed F Lifshitz, Publ Marcel Dekker, pp 167-196,
1982.
228. Cushard Jr WG Jr, Creditor M, Canterbury JM, Reiss E:
Physiologic hyperparathyroidism in pregnancy. J Clin Endocr Metab
34:767-771, 1972.
229. Martin HE, Mehl J, Wertman M: Clinical studies of
magnesium metabolism. Med Clin N.A. 36:1157-1171, 1952.
230. Gordan GS: Recent progress in calcium metabolism:
clinical application. Calif Med 114:28-43,1971.
231. Bachem MG, Strobel B, Jastram U, Janssen EG, Paschen K:
[Magnesium and Diabetes]. Magnesium Bull 2:35-39, 1980. (in
German)
232. Bachem MG, Scheffler K, Jastram U, Pfeiffer EF: [Efficacy
of oral magnesium supplementation in type I diabetics with
nocturnal leg cramps] Magnesium Bull 8:280-283, 1986. (in
German)
233. Lindeman RD, Adler S, Yiengst MJ, Beard ES: Influence of
various nutrients on urinary divalent cation excretion. J Lab
Clin Med 70:236-245, 1967.
234. Aikawa J: Effect of glucose and insulin on magnesium
metabolism in rabbits. Proc Soc Biol Med 103:363-366, 1969.
235. Lostroh AJ, Krahl ME: Magnesium, a second messenger for
insulin: ion translocation coupled to transport activity. Adv Enz
Regul 12:73-81, 1974.
236. Resnick LM, Gupta RK, Bhargava KK, Gruenspan H, Alderman
MH, Laragh JH: Cellular ions in hypertension, diabetes, and
obesity. A nuclear magnetic resonance spectroscopic study.
Hypertension 17 (6, Part 2):951-957, 1991.
237. Vir SC, Love AHG: Nutritional status of institutionalized
and non-institutionalized aged in Belfast. Am J Clin Nutr
32:1934-1947, 1979.
238. Seelig MS: Possible role of magnesium in disorders of the
aged. In Intervention in the Aging Process, eds W Regelson &
FM Sinex, Publ AR Liss Inc. New York, NY, 1983, pp279-283.
239. Johansson G: Magnesium metabolism. Studies in health,
primary hyperparathyroidism and renal stone disease. Scand J Urol
Nephrol Suppl 51:1-48, 1979.
240. Mountokalakis Th, Singhellakis PN, Alevizaki CC,
Caramanakos E, Ikkos DG: Absorption intestinale du magnesium chez
des malades en insuffisante renale chronique. Rev Franc Endocr
Clin Nutr Metab 17:229-232, 1976.
241. Schachter D, Rosen SM: Active transport of Ca45 by small
intestine and its dependence on vitamin D. Am J Physiol
196:357-362, 1959.
242. Alcock N, MacIntyre I: Interrelation of calcium and
magnesium absorption. Clin Sci 22:185-193, 1962.
243. Heaton FW, Hodgkinson A, Rose GA: Observations on the
relation between calcium and magnesium metabolism in man. Cin Sci
27:31-40, 1964.
244. Parlier R, Hioco D, LeBlanc R: [Relationship of magnesium
metabolism with that of calcium. I. A study of magnesium balance
in normal man and in osteopathies and nephropathies.] Rev Franc
Endocr Clin 4:93-135, 1963. (in French)
245. DuRuisseau JP, Marineau JM: [Calcium and magnesium
treatment of osteoporosis] Proc First Intl Sympos on Mg, 1971.
Vittel, France. Ed J Durlach, 2:175-192. (1973)
246. Spencer H, Lesniak M, Kramer L, Coffey J, Osis D: Studies
of magnesium metabolism in man. In Magnesium in Health and
Disease, eds M Cantin, MS Seelig, Spectrum, NY, NY, 1980, pp
912-919. (2nd Intl Mg Sympos, Quebec, Canada, 1976).
247. Lewis NM, Marcus MSK, Behling AR, Greger JL: Calcium
supplements and milk: effects on acid-base balance and on
retention of calcium, magnesium, and phosphorus. Am J Clin Nutr
49:527-533, 1989.
248. Clarkson EM, Warren RL, McDonald SJ, de Wardener HE: The
effect of a high intake of calcium on magnesium metabolism in
normal subjects and patients with chronic renal failure. Clin Sci
32:11-18, 1967.
249. Cunningham IJ: The influence of the level of dietary
magnesium and calcium contents of the bone, the bodies and the
blood serum of rats. N Zeal J Sci Tech15:191-198, 1933.
250. Orent ER, Kruse HD, McCollum EV: Studies on magnesium
deficiency in animals. VI. Chemical changes in the bone, with
associated blood changes resulting from magnesium deprivation. J
Biol Chem 106:573-592, 1934.
251. Watchorn E, McCane RA: Subacute magnesium deficiency in
rats. Biochem J 31: 1379-1390, 1937.
252. U.S. Dept Agric Nationwide Consumption Survey, 1977-1978
U.S.D.A. Sci & Educ Administration, 1980.
253. Lakshmanan FL, Rao RB, Kim WW, Kelsay JL: Magnesium
intakes, balances, and blood levels of adults consuming
self-selected diets. Am J Clin Nutr 40:1380-1389, 1984.
254. Morgan KJ, Stampley GL, Zabik ME, Fischer DR: Magnesium
and calcium dietary intakes of the U.S. population. J Am Coll
Nutr 4:195-206, 1985.
255. Spillman, DM: Calcium, magnesium and calorie intake and
activity levels of healthy adult women. J Am Coll Nutr 6:454,
1987.
256. Morgan KJ, Stampley GL: Dietary intake levels and food
sources of magnesium and calcium for selected segments of the US
population. Magnesium 7:225-233, 1988
257. Abdulla M, Behbehani A, Dashti H: Marginal deficiency of
magnesium and the suggested treatment. In Magnesium in Health and
Disease. eds Y Itokawa, J Durlach, Publ J. Libbey, London, 1989
(5th Intl Mg Sympos, Kyoto, 1988), pp 111-117.
258. Pennington JA, Young BE, Wilson DB: Nutritional elements
in U.S. diets; results from the Total DietStudy, 1982 to 1986. J
Am Diet Assoc 89:659-664, 1989.
259. Tsang RC: Neonatal magnesium disturbances. Am J Dis Child
124:282-293, 1972.
260. Paunier L, Radde IC, Kooh SW, Conen PEE, Fraser D:
Primary hypomagnesemia with secondary hypocalcemia in an infant.
Pediatrics 41:385-402, 1968.
261. Paunier L: Magnesium malabsorption. Adv in Intern Med
& Pediatr 42:113- 131, 1979.
262. Mimouni F, Loughead J, Miodovnik M, Khoury J, Tsang RC:
Early neonatal predictors of neonatal hypocalcemia in infants of
diabetic mothers: an epidemiologic study. Am J Perinatol
7:203-206, 1990.
263. Turner TL, Cockburn F, Forfar JO: Magnesium therapt in
neonatal tetany. Lancet 1:282-284, 1977.
264. Heaton FW, Parsons FM: Metabolic effect of high magnesium
intake. Clin Sci 21:273-284,1961.
265. Estep H, Shaw WA, Watlington C, Hobe R, Holland W, Tucker
SG: Hypocalcemia due to hypomagnesemia and reversible parathyroid
hormone responsiveness. J Clin Endocrinol Metab 29:842-848,
1969.
266. Anast CS, Winnacker JL, Forte LR, Burns TW: Impaired
release of parathyroid hormone in magnesium deficiency. J Clin
Endocr Metab 42:707- 720, 1976.
267. Suh SM, Tashjian Jr AH, Matsuo N, Parkinson DK, Fraser D:
Pathogenesis of hypocalcemia in primary hypomagnesemia: normal
end-organ responsiveness to parathyroid hormone, impaired
parathyroid gland function. J Clin Invest 52:153-160, 1973.
268. Rude RK, Oldham SB, Singer FR: Functional
hypoparathyroidism and parathyroid hormone end-organ resistance
in human magnesium deficiency. Clin Endocr 5:209-224, 1976.
269. Rude RK, Oldham SB, Sharp CF, Singer F: Parathyroid
hormone secretion in magnesium deficiency. J Clin Endocr Metab
47:800-806, 1978.
270. Rude RK, Oldham SB: Hypocalcemia of Mg deficiency:
altered modulation of adenylate cyclase by Mg++ and Ca++ may
result in impaired PTH secretion and PTH end-organ resistance. In
Magnesium in Cellular Processes and Medicine, Eds BM Altura,
JDurlach, MS Seelig, Publ Karger, Basel, Switzerland, pp 183-195,
1987.
271. Norman AW, Henry H: 1,25-dihydroxycholecalciferol - A
hormonally active form of vitamin D3. Rec Progr Hormone Res
30:431-480, 1974.
272. DeLuca HF: Vitamin D endocrinology. Ann Intern Med
85:367-377, 1976.
273. McGeown M: Sex, age, and hyperparathyroidism. Lancet
1:887-888, 1969.
274. Muller H: Sex, age and hyperparathyroidism. Lancet
1:449-450, 1969.
275. Hossain M, Smith DA, Nordin BEC: Parathyroid activity and
menopausal osteoporosis. Lancet 1:809-811, 1970.
276. Ranney R: Antagonism between estrone and parathyroid
extract in their effects upon bone structure. Endocrinology
64:594-601, 1959.
277. Atkins D, Zanelli JM, Peacock M, Nordin BEC: The effect
of oestrogens on the response of bone to parathyroid hormone in
vitro. J Endocr 54:107- 117, 1972.
278. Orimo H, Fujita T, Yoshikawa M: Increased sensitivity of
bone to parathyroid hormone in ovariectomized rats. Endocrinology
90: 760-763, 1972.
279. Heaney RP: Prevention of osteoporotic fracture in women.
In The Osteoporotic Syndrome. LV Avioli, publ Grune &
Stratton Inc. Orlando, FL, 1987, 67-90.
280. Riggs BL, Jowsey J, Kelly PJ, Jones JD, Maher FT: Effect
of sex hormones on bone in primary osteoporosis. Clin Invest
48:1065-1072, 1969.
281. Larvor P, Girard A, Brochart M, Parodi A, Sevestre J:
[Study of experimental Mg deficiency in the calf..I Clinical,
biochemical, and pathologic observations.] Ann Biol Anim Bioch
Biophys 4:345-369, 1964. (in French)
282. Care AD, Sherwood LM, Potts Jr JT, Aurbach GD: Perfusion
of the isolated parathyroid gland of the goat and sheep. Nature
209:55-57. 1967.
283. Buckle RM, Care AD, Cooper CW, Gitelman HJ: The influence
of plasma magnesium concentration on parathyroid Hormone
secretion. J Endocr 42:529-534, 1968.
284. Targovnik JH, Rodman JS, Sherwood LM: Regulation of
parathyroid hormone secretion in vitro; quantitative aspects of
calcium and magnesium ion control. Endocrinol 88:1477-1482,
1971.
285. Gallagher JC, Riggs BL, Eisman J, Hamstra A, Arnaud SB,
DeLuca HF: Intestinal calcium absorption and serum vitamin
metabolites in normal subjects and osteoporotic patients. J Clin
Invest 64:729-736, 1979.
286. Slovik DM, Adams SJ, Neer RM, Holick MF, Potts Jr JT:
Deficient production of 1,25-dihydroxyvitamin D in elderly
osteoporotic patients. N Engl J Med 305:372-374, 1981.
287. Tsai K-S, Heath III H, Kumar R, Riggs BL: Impaired
vitamin D metabolism with aging in women. Possible role in
pathogenesis of senile osteoporosis. J Clin Invest 73:1668-1672,
1984.
288. Riggs BL, Melton III LJ: Involutional osteoporosis. N
Engl J Med 314: 1676-1686, 1986.
289. Reichel H, Koeffler HP, Norman AN: The role of the
vitamin D endocrine system in health and disease. N Engl J Med
320:980-991, 1989.
290. Silverberg SJ, Shane E, de la Cruz L, Segre GV, Clemens
TL, Bilezikian JP: Abnormalities in parathyroid hormone secretion
and 1,25-dihydroxyvitamin D3 formation in women with
osteoporosis. N Engl J Med 320:277-281, 1989.
291. Franz KB: Abnormalities in parathyroid hormone secretion
and 1,25-dihydroxyvitamin D3 formation in women with
osteoporosis. N Engl J Med 320:1697-1698, 1989.
292. Kumar R, Cohen WR, Silva P, Epstein FH: Elevated
1,25-dihydroxyvitamin D plasma levels in normal human pregnancy
and lactation. J Clin Invest 63: 342-344, 1979.
293. Gallagher JC, Riggs BL, DeLuca HF: Effect of estrogen on
calcium absorption and serum vitamin D metabolites in
postmenopausal osteoporosis. J Clin Endocrinol Metabol
51:1359-1364, 1980.
294. Cheema C, Grant BF, Marcus R: Effects of estrogen on
circulating "free" and total 1,25-dihydroxyvitamin D and on the
parathyroid-vitamin D axis in postmenopausal women. J Clin Invest
83:537-542, 1989.
295. Rude RK, Adams JS, Ryzen E, Endres DB, Nimni H, Horst RL,
Haddad Jr JG, Singer FR: Low serum concentrations of
1,25-dihydroxyvitamin D in human magnesium deficiency. J Clin
Endocr Metab 61:933-940, 1985.
296. Fuss M, Cogan E, Gillet C: Magnesium administration
reverses the hypocalcemia secondary to hypomagnesemia despite low
circulating levels of 25-hydroxyvitamin D and 1,25-dihydroxy
vitamin D. Clin Endocrinol 22:807-815, 1985.
(This article has been placed on this web site with the
permission of the Journal of the American College of Nutrition.
We thank Dr. Mildred S. Seelig for providing us the information
on a diskette.)
This page was first uploaded to The Magnesium Web Site on
October 16, 1995
http://www.mgwater.com/